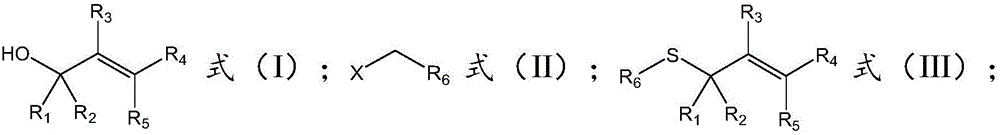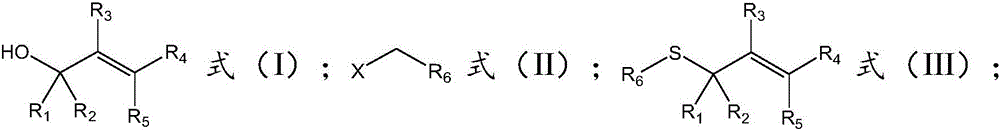Synthetic method of asymmetrical thioether
A synthetic method and asymmetric technology, applied in the formation/introduction of thiol/thioether group, thioether preparation, chemical instruments and methods, etc., can solve problems such as restricting practical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a kind of synthetic method of unsymmetrical thioether, comprises the following steps:
[0035] a), under the catalytic conditions of tetrabutylammonium halide, the compound of formula (I), the compound of formula (II) and oxysulfide are reacted in a solvent to obtain the asymmetric thioether with the structure of formula (III);
[0036]
[0037] Among them, R 1 selected from phenyl, substituted phenyl, naphthyl, substituted naphthyl, thienyl or substituted thienyl, R 2 selected from hydrogen, phenyl, substituted phenyl, naphthyl, substituted naphthyl, thienyl, or substituted thienyl; or R 1 , R 2 Form a fluorene ring or a thioxanthene ring with the C connected to it;
[0038] R 3 selected from hydrogen or alkyl;
[0039] R 4 selected from hydrogen, phenyl, substituted phenyl, naphthyl, substituted naphthyl, thienyl or substituted thienyl, R 5 selected from hydrogen; or R 4 , R 5 Form a fluorene ring or a thioxanthene ring with the C conn...
Embodiment 1
[0066] Synthesis of 4-((3,3-diarylallylthio)methyl)benzonitrile
[0067] Weigh 0.3mmol 1,1-diphenylprop-2-enyl-1-ol (0.0631 grams), 0.6mmol p-cyanobenzyl chloride (0.0910 grams), 0.72mmol sodium thiosulfate (0.1138 grams), 0.06 Mmol tetrabutylammonium iodide (0.0222 grams), in a 20mL test tube reaction tube, add 1mL water as a solvent, seal and seal, stir and react at 80°C for 5 hours; Sodium sulfate water drying and column chromatography separation (column chromatography separation conditions: the stationary phase is 300-400 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B ) was 1:20→1:6), and 0.0778 gram of reaction product was obtained.
[0068] The above-mentioned reaction product is characterized, and the result is:
[0069] 1 H NMR (400MHz, CDCl 3 ):δ=7.49–7.42(m,2H),7.38–7.33(m,3H),7.29–7.25(m,3H),7.22–7.18(m,2H),7.18–7.13(m,4H),6.08 (t, J=7.8Hz, 1H), 3.63(s, 2H), 3.18(d, J=7.8Hz, 2H)ppm;
...
Embodiment 2
[0072] Synthesis of 4-((3,3-bis(4-fluorophenyl)allylthio)methyl)benzonitrile
[0073] Weigh 0.3mmol 1,1-bis(4-fluorophenyl)prop-2-enyl-1-ol (0.0739 grams), 0.6mmol p-cyanobenzyl chloride (0.0910 grams), 0.72mmol sodium thiosulfate ( 0.1138 grams), 0.06mmol tetrabutylammonium iodide (0.0222 grams), in a 20mL test tube reaction tube, add 1mL water as a solvent, seal and seal, stir and react at 80°C for 5 hours; after the reaction, the reaction solution is passed through Ethyl acetate, anhydrous sodium sulfate drying and column chromatography separation (column chromatography separation conditions: the stationary phase is 300-400 mesh silica gel powder, the mobile phase is ethyl acetate (A) and petroleum ether (B), the mobile phase changes The procedure (A:B) is 1:20 → 1:6), yielding 0.0932 g of reaction product.
[0074] The above-mentioned reaction product is characterized, and the result is:
[0075] 1 H NMR (400MHz, CDCl 3 ):δ=7.51(d, J=8.3Hz, 2H), 7.24(d, J=8.2Hz, 2H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com