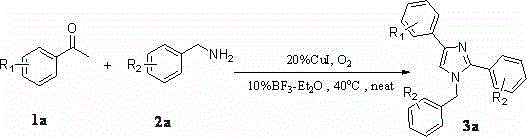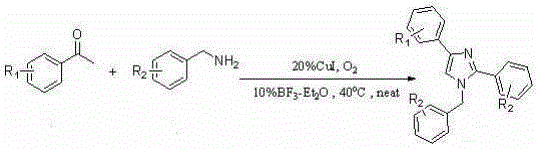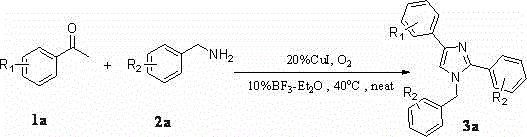A kind of synthetic method of many substituted imidazoles
A synthesis method and multi-substitution technology, applied in the field of synthesis of multi-substituted imidazoles, can solve the problems of less synthesis methods, difficult preparation of reaction substrates, and toxicity of catalysts, and achieve high yield, convenient post-treatment, and catalytic conditions simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment one: Synthesis of 1-Benzyl-2,4-Diphenylimidazole
[0043] Weigh 2mmol of acetophenone (0.242g), 3mmol of amine (0.642g), 0.04mmol of cuprous iodide (0.076g), and 0.02mmol of boron trifluoride ether (0.028g) in a 25mL Schlenk-tube, Stirring at 40° C. for 24 hours in an oxygen atmosphere, separated by column chromatography to obtain 0.442 g of pure 1-benzyl-2,4-diphenylimidazole with a yield of 71%.
Embodiment 2
[0044] Embodiment two: Synthesis of 1-benzyl-2-phenyl-4-p-fluorophenylimidazole
[0045] Weigh 2mmol of p-fluoroacetophenone (0.276g), 3mmol of amine (0.642g), 0.04mmol of cuprous iodide (0.076g), 0.02mmol of boron trifluoride ether (0.028g) in a 25mL Schlenk-tube , stirred at 40°C for 24 hours in an oxygen atmosphere, and separated by column chromatography to obtain 0.327 g of pure 1-benzyl-2-phenyl-4-p-fluorophenylimidazole, with a yield of 50%.
Embodiment 3
[0046] Embodiment three: Synthesis of 1-benzyl-2-phenyl-4-p-chlorophenylimidazole
[0047] Weigh 2mmol of p-chloroacetophenone (0.3092g), 3mmol of amine (0.642g), 0.04mmol of cuprous iodide (0.076g), 0.02mmol of boron trifluoride ether (0.028g) in a 25mL Schlenk-tube , stirred at 40°C for 24 hours in an oxygen atmosphere, and separated by column chromatography to obtain 0.368 g of pure 1-benzyl-2-phenyl-4-p-chlorophenylimidazole, with a yield of 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com