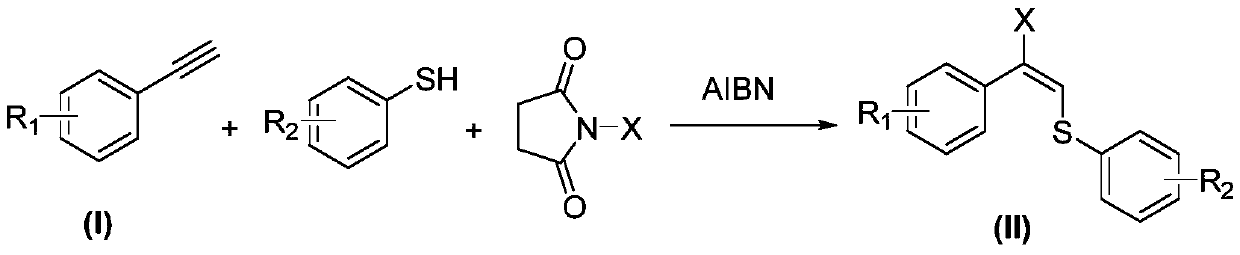
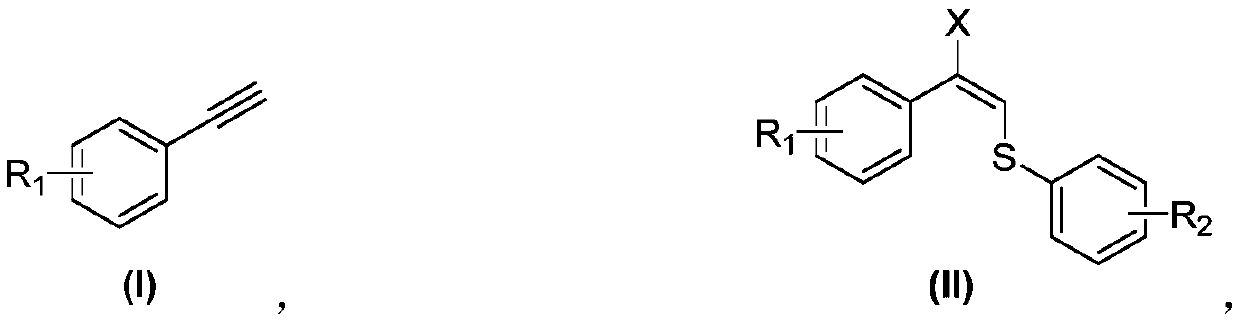Synthetic method of allyl halosulfide
A technique for the synthesis of allyl halides, which is applied in the field of organic synthesis, can solve the problems of narrow substrate range, low yield, and preparation of reagents in advance, and achieve simple and reasonable process conditions, high chemical selectivity, and good conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 25mL Teflon tube, add 20.4mg (0.2mmol) phenylacetylene, 6.6mg (0.04mmol) azoisobutyronitrile, 27.3mg (0.22mmol) 4-methylthiophenol and 49.5mg (0.22mmol) N -Iodosuccinimide, then add 1mL of acetonitrile, heat to 80°C and stir for 12h. After the reaction, the solvent was distilled off from the reaction liquid under reduced pressure to obtain a crude product, and finally the mixture of ethyl acetate and petroleum ether with a volume ratio of 1:20 was used as an eluent, and trans-1-( Phenyl)-1-(iodovinyl)-4-chlorophenylsulfide 45.4 mg, yield 61%.
[0026] 1 H NMR (500MHz, CDCl 3 )δ7.45(dt, J=7.3,2.6Hz,2H),7.40(d,J=8.4Hz,1H),7.34(dd,J=8.5,6.8Hz,2H),7.32-7.28(m,2H ), 7.23(d, J=7.2Hz, 2H), 7.04-7.01(m, 1H);
[0027] GCMS(EI)Calcd.for C 14 h 10 ClIS 371.92, found 371.95.
Embodiment 2
[0029] In a 25mL Teflon tube, add 23.2mg (0.2mmol) 4-methylphenylacetylene, 6.6mg (0.04mmol) azoisobutyronitrile, 24.2mg (0.22mmol) thiophenol and 29.3mg (0.22mmol) N - Chlorosuccinimide, then add 1 mL of acetonitrile, heat to 80 ° C and stir for 16 h. After the reaction, the solvent was distilled off from the reaction liquid under reduced pressure to obtain a crude product, and finally the mixture of ethyl acetate and petroleum ether with a volume ratio of 1:20 was used as an eluent, and trans-1-( 4-methylphenyl)-1-(chlorovinyl)-phenylene sulfide 30.2 mg, yield 58%.
[0030] 1 H NMR (500MHz, CDCl 3 )δ7.38-7.33(m,2H),7.20(dd,J=5.1,2.0Hz,3H),7.11-7.06(m,2H),6.92(d,J=7.9Hz,2H),6.52(s ,1H),2.21(s,3H);
[0031] GCMS(EI)Calcd.for C 15 h 13 ClS 260.04, found 260.09.
Embodiment 3
[0033] Add 36.0mg (0.2mmol) 4-bromophenylacetylene, 6.6mg (0.04mmol) azoisobutyronitrile, 27.3mg (0.22mmol) 4-chlorothiophenol and 39.2mg (0.22mmol) to a 25mL Teflon tube ) N-bromosuccinimide, then add 1 mL of acetonitrile, heat to 80 ° C and stir for 24 h. After the reaction, the solvent was distilled off from the reaction liquid under reduced pressure to obtain a crude product, and finally the mixture of ethyl acetate and petroleum ether with a volume ratio of 1:20 was used as an eluent, and trans-1-( 4-bromophenyl)-1-(bromovinyl)-4-bromophenylene sulfide 30.2 mg, yield 60%.
[0034] 1 H NMR (500MHz, CDCl 3 )δ7.43-7.33 (m, 4H), 7.30 (d, J = 8.2Hz, 3H), 7.19 (s, 1H), 6.77 (s, 1H);
[0035] GCMS(EI)Calcd.for C 14 h 9 BrClS 401.85, found 401.89.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com