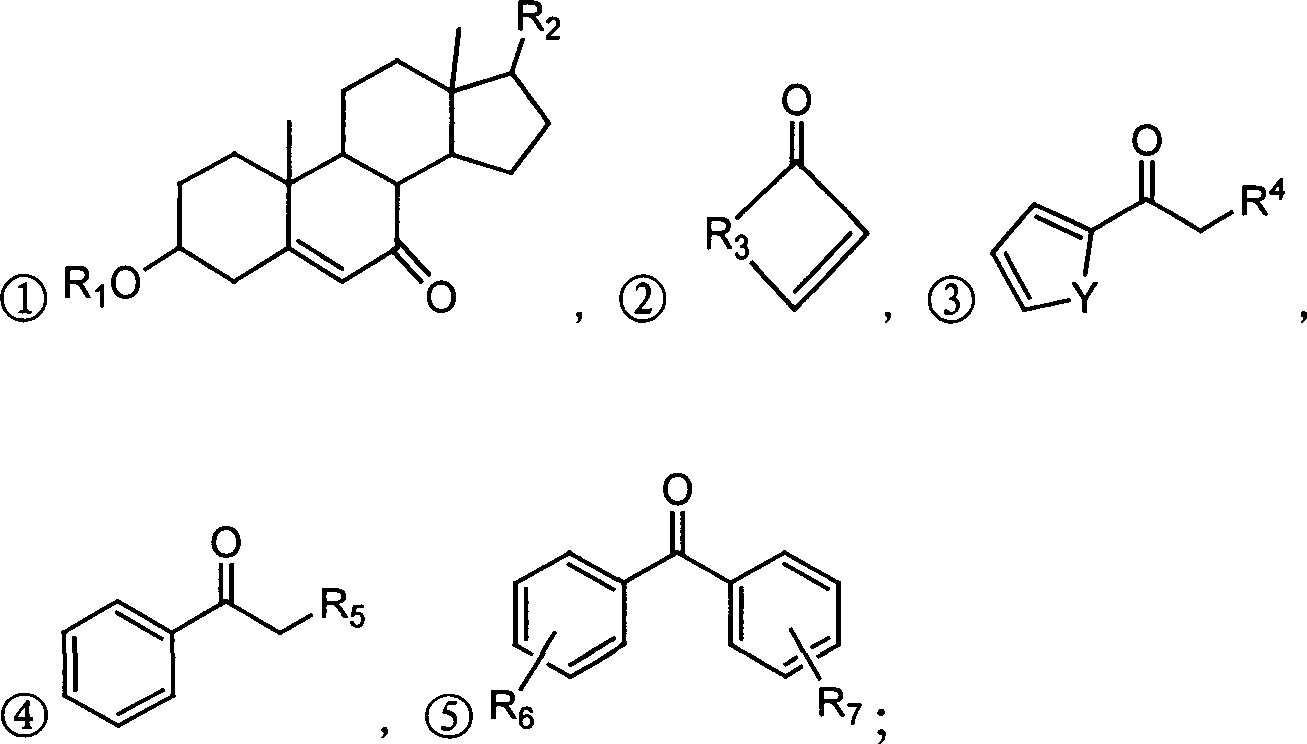
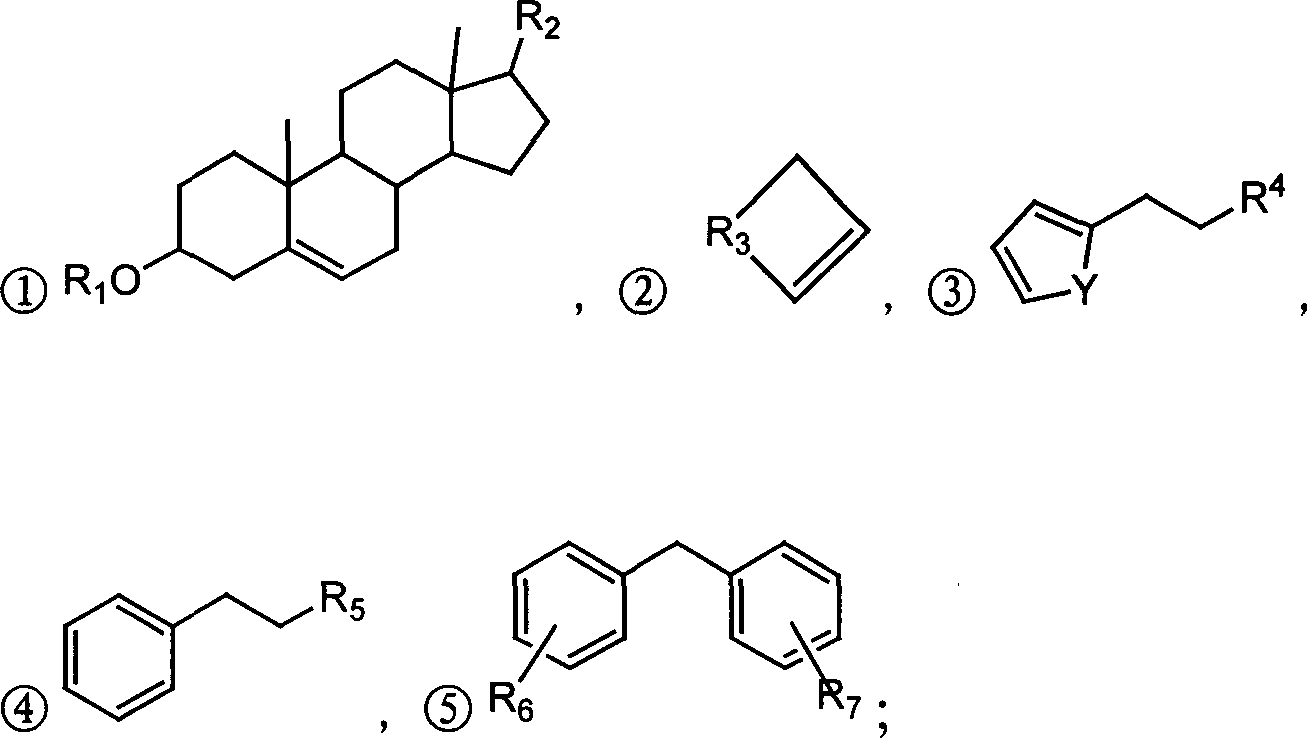
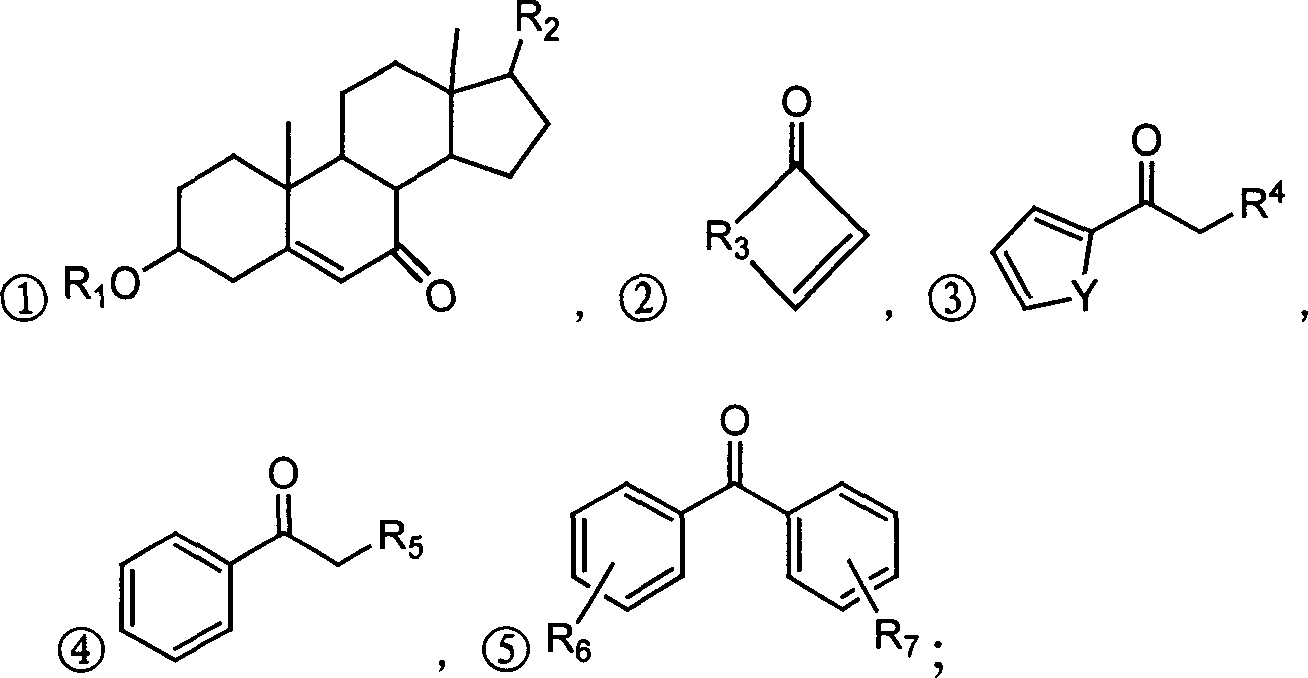
Alpha,beta-unsaturated ketone or arone environment-friendly synthesis method
A green synthesis and unsaturated technology, applied in the field of α, can solve the problems of large amount of catalyst, easy to cause pollution, difficult post-processing, etc., and achieve the effects of low production cost, pollution prevention, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In a 2500ml four-neck flask equipped with a thermometer and mechanical stirring, add 168g (1mol) of diphenylmethane, 60g of N-hydroxysuccinimide, azobisisobutylcyanide (AIBN, 0.5g), and use 1800ml of acetic acid Ethyl ester / butanone (V / V=1:1) mixed solvent was dissolved and stirred, heated to 70°C, and air was introduced at V=1.5ml / min. The reaction temperature was maintained and stirred for 12 hours. Cool to below 0°C, and fully stir for 30 minutes, then stand still for 10 minutes, and filter to obtain 55 g of catalyst for filter cake recovery. The filtrate was transferred to a 2500ml three-necked flask, 10ml of triethylamine and 15g of ferrous sulfate were added, heated to 70°C and stirred for 24 hours. Cool to room temperature, add 5% dilute hydrochloric acid to make the pH value ≤ 5, separate the liquids, wash the upper transparent solution with water and concentrate to obtain a light yellow solid, which is recrystallized with 80% ethanol to obtain 166g of pure pro...
Embodiment 2
[0036] In a 2500ml four-necked bottle equipped with a thermometer and mechanical stirring, add 168g (1mol) of diphenylmethane, 60g of N-hydroxymaleimide, azobisisobutylcyanide (AIBN, 0.5g), and use 1800ml of acetic acid Ethyl ester / butanone (V / V=1:1) mixed solvent was dissolved and stirred, heated to 70°C, and air was introduced at V=1.5m1 / min. The reaction temperature was maintained and stirred for 12 hours. Cool to below 0°C, and fully stir for 30 minutes, then stand still for 10 minutes, and filter to obtain 52.5 g of catalyst for filter cake recovery. The filtrate was transferred to a 2500ml three-necked flask, 10ml of triethylamine and 15g of ferrous sulfate were added, heated to 70°C and stirred for 24 hours. Cool to room temperature, add 5% dilute hydrochloric acid to make the pH value ≤ 5, separate the liquids, wash the upper transparent solution with water and concentrate to obtain a light yellow solid, which is recrystallized with 80% ethanol to obtain 155g of pure ...
Embodiment 3
[0038]In a 2500ml four-necked bottle equipped with a thermometer and a mechanical stirrer, add 168g (1mol) of diphenylmethane, 85g of N-hydroxyphthalimide, and azobisisobutylcyanide (AIBN, 0.5g). 1800ml of ethyl acetate / butanone (V / V=1:1) mixed solvent was dissolved and stirred, heated to 70°C, and air was introduced at V=1.5ml / min. The reaction temperature was maintained and stirred for 12 hours. Cool to below 0°C, and fully stir for 30 minutes, then stand still for 10 minutes, and filter to obtain 82.5 g of catalyst for filter cake recovery. The filtrate was transferred to a 2500ml three-necked flask, 10ml of triethylamine and 15g of ferrous sulfate were added, heated to 70°C and stirred for 24 hours. Cool to room temperature, add 5% dilute hydrochloric acid to make the pH value ≤ 5, separate the liquids, wash the upper transparent solution with water and concentrate to obtain a light yellow solid, which is recrystallized with 80% ethanol to obtain 146g of pure product with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com