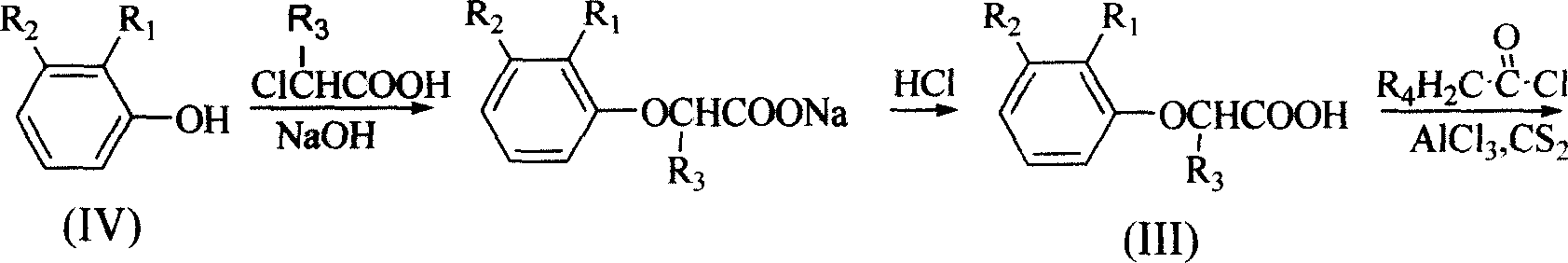Alpha, beta-unsaturated ketone compound and its prepn process and GST-Pi inhibiting activity
A ketone compound and unsaturated technology, which is applied in the field of organic compound synthesis and pharmaceutical application, can solve the problems of low activity of ethacrynic acid, restricting clinical application, large toxic and side effects, etc. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1.2-(2-methylphenoxy group) propionic acid (III)
[0036] Dissolve sodium hydroxide (15g, 375mmol) in 20ml of water, add it to a 250ml round bottom flask, stir at room temperature, add o-cresol (10.8, 100mmol), and add 2-chloropropionic acid (18.4g , 170mmol), reacted at 85-90°C for 2 hours, cooled, added 200ml of water, filtered, and the filtrate was acidified to pH 2.0 with concentrated hydrochloric acid to produce a brown oil, which was extracted with ether (100ml×2), and the ether solution was washed with 5% NaHCO 3 Extract (75ml×2), NaHCO 3 The extract was acidified with concentrated hydrochloric acid to pH 2.0 to produce a solid, which was filtered and dried to obtain 2-(2-methylphenoxy)propionic acid, yellow powder, yield 32.2%, mp: 86-89°C, TLC Rf=0.49 (n-hexane / ethyl acetate 2:1). 1 H-NMR (DMSO-d 6 )δ: 12.9(s, 1H), 7.13(d, J=7.3Hz, 1H), 7.10(t, J=7.7Hz, 1H), 6.83(t, J=7.3Hz, 1H), 6.75(d, J=8.2Hz, 1H), 4.78(q, J=6.7Hz, 1H), 2.18...
Embodiment 2
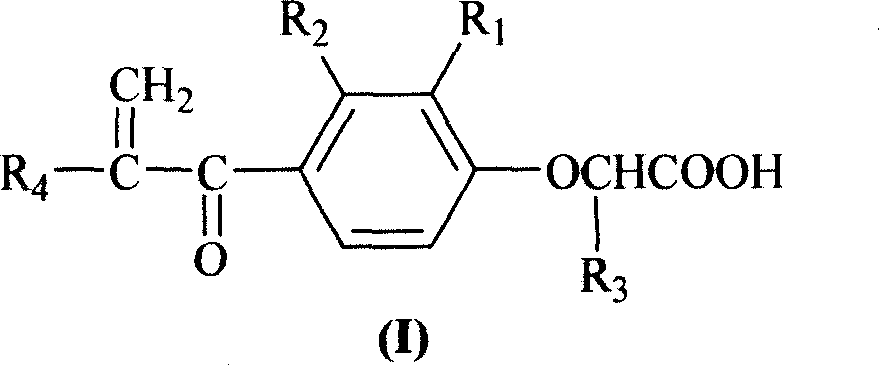
[0046] Example 2. Preparation of 2-[2-methyl-4-(1-oxygen-propyl)phenoxy]propionic acid (II)
[0047] 2-(2-Methylphenoxy)propanoic acid (4.3 g, 24 mmol) was added to CS 2 (70ml), stirred at room temperature, added anhydrous aluminum trichloride powder (10g, 75mmol) in batches, and then slowly added propionyl chloride (2.6ml, 30mmol) dropwise to produce a yellow viscous substance, reacted at 55-60°C for 4 hours , cooling, the CS 2 Pour out, add 100g of ice water containing 3ml of concentrated hydrochloric acid, stir, produce oil, extract with ether (100ml×2), ether solution with 5% NaHCO 3 Extract (50ml×2), NaHCO 3 The extract was acidified to pH 2.0 with concentrated hydrochloric acid to produce a precipitate, which was filtered and dried to obtain 2-[2-methyl-4-(1-oxo-propyl)phenoxy]propionic acid as a yellow powder with a yield of 72.7% , mp: 129~131°C, TLC Rf=0.33 (n-hexane / ethyl acetate 2:1). 1 H-NMR (DMSO-d 6 )δ: 13.05(s, 1H), 7.84(m, 2H), 6.92(m, 1H), 5.01(q, J=6.7Hz...
Embodiment 3
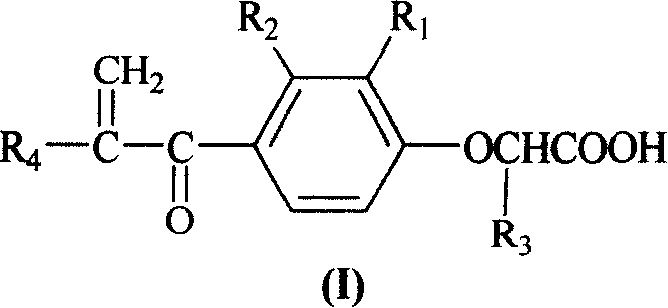
[0063] Example 3. Preparation of 2-[2-methyl-4-(2-methylene-1-oxygen-propyl)phenoxy]propionic acid (I)
[0064] Dissolve 2-[2-methyl-4-(1-oxo-propyl)phenoxy]propionic acid (4.0g, 17mmol) in ethanol (34ml), add 30% formaldehyde (1.7ml, 17mmol), shake Evenly, K 2 CO 3 (2.3g, 17mmol) was dissolved in a mixed solution of water (17ml) and ethanol (10ml), and these two solutions were slowly added to refluxing ethanol (24ml) at the same time, reacted at 85-90°C for 3 hours, cooled, The product was poured into hydrochloric acid solution (5ml concentrated hydrochloric acid, 340ml water) to produce light yellow oil, which was extracted with diethyl ether (150ml×2), evaporated under reduced pressure to remove diethyl ether, separated by column chromatography, eluent petroleum ether: chloroform: methanol =10:10:1, 2-[2-methyl-4-(2-methylene-1-oxygen-propyl)phenoxy]propionic acid was obtained, brown oil, yield 28.6%, TLC Rf= 0.54 (petroleum ether / acetone 2:1). 1 H-NMR (DMSO-d 6 )δ: 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com