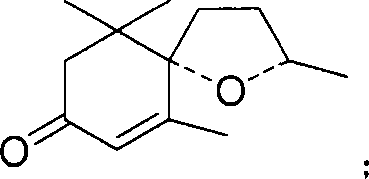
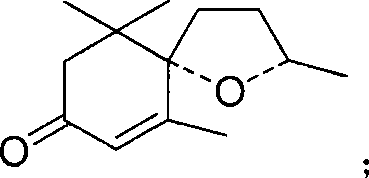
Allylic oxidation method for cyclohexene derivative
A technology for allylic oxidation and cyclohexene, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of lengthy synthesis process and low yield Human satisfaction, unfriendly environment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Synthesis of oxyisophorone
[0031] α-isophorone 1.38g, VO(acac) 2 0.20g, 20ml of acetone, react with 15ml of tert-butyl hydroperoxide (70%) at 50℃, stir after 18h, remove the catalyst, rotary evaporate, evaporate the solvent and tert-butanol to dryness, extract with 2*20ml of ether, Na 2 SO 3 Solution 20ml for washing, water 20ml for washing, anhydrous Na 2 SO 4 Drying, rotary evaporation, petroleum ether: ethyl acetate = 8:1 as the eluent, column chromatography to obtain 0.6 g (40%) oxyisophorone.
[0032] NMR 1 H(CDCl 3 , 300MHz): 1.249(s, 6H), 2.014(s, 3H), 2.723(s, 2H). 6.555-6.571(m, 1H). MS: 152(M + , 66%), 137 (11%), 124 (4%), 109 (16%), 96 (96%), 81 (5%), 68 (100%).
Embodiment 2
[0033] Embodiment 2: Synthesis of theaspiranone
[0034] Theaspirane 1.94g, VO(acac) 2 0.266g, in 15ml of acetonitrile, react with 7ml of tert-butyl hydroperoxide (70%) at 40°C, stop the reaction after 6h, remove the catalyst, rotary evaporate, evaporate the solvent and tert-butanol to dryness, extract with 2*20ml of ether, Na 2 SO 3 Solution 20ml for washing, water 20ml for washing, anhydrous Na 2 SO 4 Drying, rotary evaporation, the peak area of theaspiranone was 70% after GC-MS analysis. MS: 208(M + , <1%), 152 (100%), 110 (63%), 193 (1%), 165 (2%), 96 (9%), 82 (8%), 69 (7%).
Embodiment 3
[0035] Embodiment 3: Synthesis of α-pinenone
[0036] α-pinenone 1.36g, VO(acac) 2 0.266g, 20ml of acetone, react with 7ml of tert-butyl hydroperoxide (70%), stop the reaction for 10h, remove the catalyst, rotary evaporate, evaporate the solvent and tert-butanol to dryness, extract with 2*20ml of ether, Na 2 SO 3 Solution 20ml for washing, water 20ml for washing, anhydrous Na 2 SO 4 Drying, rotary evaporation, petroleum ether: ethyl acetate = 5:1 as eluent, column chromatography to obtain α-pinenone 0.93g (62%).
[0037] MS: 150(M + , 48%), 135 (79%), 122 (17%), 107 (100%), 95 (13%), 91 (59%), 79 (36%), 67 (15%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com