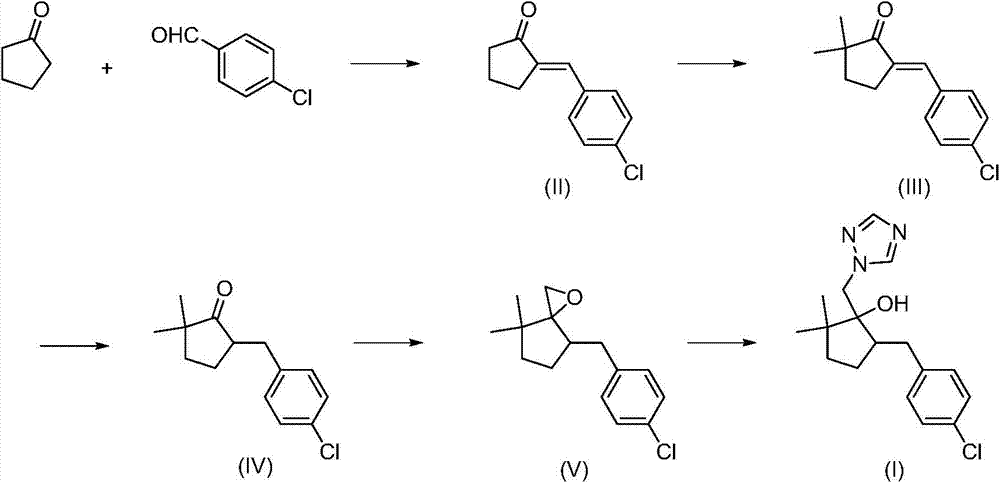
High purity metconazole and preparation method thereof
A metconazole and high-purity technology, which is applied in the field of high-purity metconazole and its preparation, can solve the problems of unobtainable raw materials, poor economy, long route and the like, and achieves short route, high yield and good atom economy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of embodiment 1,2-(4-chlorobenzylidene) cyclopentanone (II)
[0062] In a dry 150mL single-necked bottle, add pyrrolidine (1mL, 0.0078mol, 0.1eq), add tetrahydrofuran (70mL), cyclopentanone (6.9mL, 0.078mol, 1.0eq), p-chlorobenzaldehyde (11.0g, 0.078mol , 1.0eq), stirred at 25°C for 6 hours. Ethyl acetate was added for extraction and liquid separation. After spin-drying, the target product was obtained with a yield of 68%.
[0063] 1 H NMR (400MHz, CDCl 3 ): δ7.45-7.47(d, 2H), 7.37-7.39(d, 2H), 7.32-7.33(t, 1H), 2.93-2.97(td, 2H), 2.39-2.43(t, 2H), 2.01 -2.08(p, 2H).
Embodiment 2
[0064] The synthesis of embodiment 2,2-(4-chlorobenzylidene) cyclopentanone (II)
[0065] In a dry 250mL single-necked bottle, add KOH (2.2g, 0.039mol, 0.5eq), add water (95mL), stir to dissolve, add dichloromethane (70mL), cyclopentanone (6.9mL, 0.078mol, 1.0eq) . p-Chlorobenzaldehyde (13.2g, 0.094mol, 1.2eq), stirred at 25°C for 12 hours. Ethyl acetate was added for extraction and liquid separation. After spin-drying, the target product was obtained with a yield of 85%.
Embodiment 3
[0066] The synthesis of embodiment 3,2-(4-chlorobenzylidene) cyclopentanone (II)
[0067] In a dry 150ml single-necked bottle, add methanesulfonic acid (50μL, 0.00078mol, 0.01eq), add dimethyl sulfoxide (70mL), cyclopentanone (6.9mL, 0.078mol, 1.0eq), p-chlorobenzaldehyde (8.8 g, 0.062mol, 0.8eq), reflux (189°C) and stir for 1 hour. Ethyl acetate was added for extraction and liquid separation. After spin-drying, the target product was obtained with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com