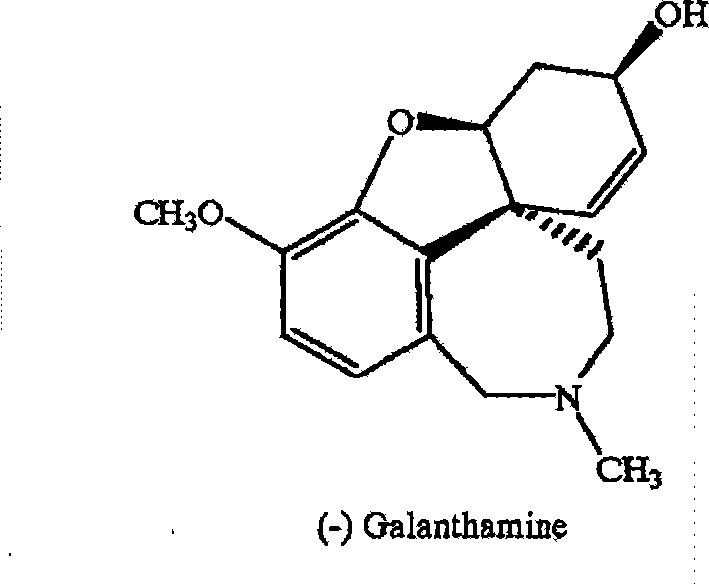
Chiral synthesis method for (-)-galantamin hydrobromide
A technology of galantamine hydrobromide and synthesis method, which is applied in the direction of asymmetric synthesis, organic chemical method, chemical instrument and method, etc., and can solve the problems of large environmental impact, difficulty of obtaining, unsuitable for large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
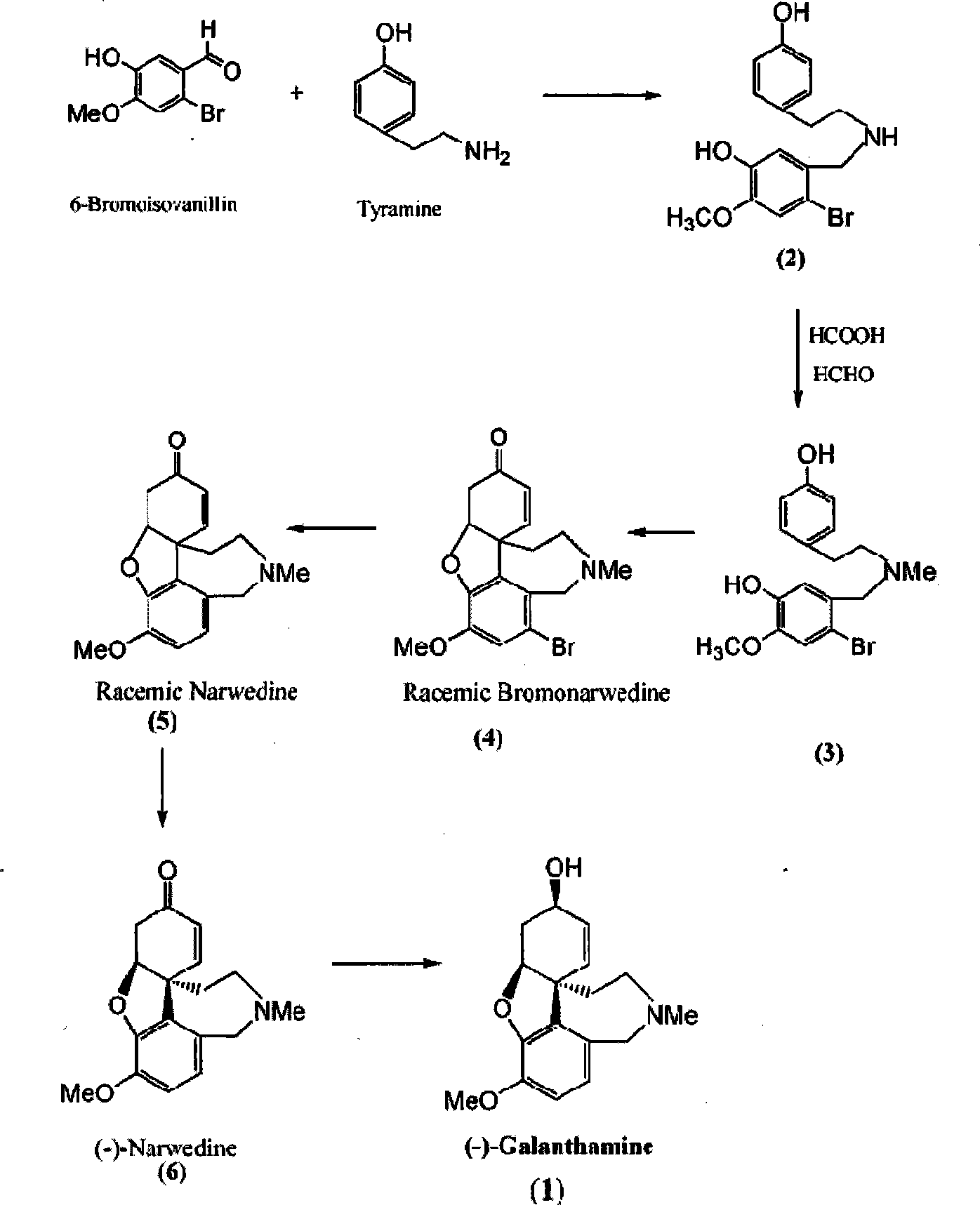
[0024] N-(2-Bromo-5-hydroxy-4-methoxybenzyl)-4-hydroxyphenethylamine (2)
[0025] To a solution of 65 g (0.28 mol) of 6-bromoisovanillin dissolved in 300 ml of methanol, a (methanol) solution of 38 g (0.28 mol) of tyramine was added dropwise at room temperature.
[0026] The mixture was stirred for 5h.
[0027] Cool to 0°C, add NaBH in small portions 4 13.4g (0.34mol) (minimal foaming is preferred).
[0028] Then the reaction solution was stirred at room temperature for 5 h. (Tracking TLC: EtOAc / MSO, 1 / 3)
[0029] Methanol was distilled off under reduced pressure, and the oily residue was dissolved in ethyl acetate, washed with brine, and dried over anhydrous magnesium sulfate.
[0030] Reduced pressure to dryness gave 82.3 g (83.1%) of yellow oil 2 (as HCl salt, mp 229-230°C)
[0031] N-(2-Bromo-5-hydroxy-4-methoxybenzyl)-4-hydroxyphenethylmethylamine (3)
[0032] 250g (0.14mol) is dissolved in a mixture of 98%-100% formic acid 50ml and 36%-38% formaldehyde 50ml. Heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com