Method for analyzing biological samples by using matrix assisted laser desorption ionization-Fourier transform ion cyclotron resonance mass spectra
A technology of ion cyclotron resonance and matrix-assisted laser, which is applied to the analysis of materials, the preparation of test samples, and the analysis of materials by electromagnetic means, and can solve problems that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
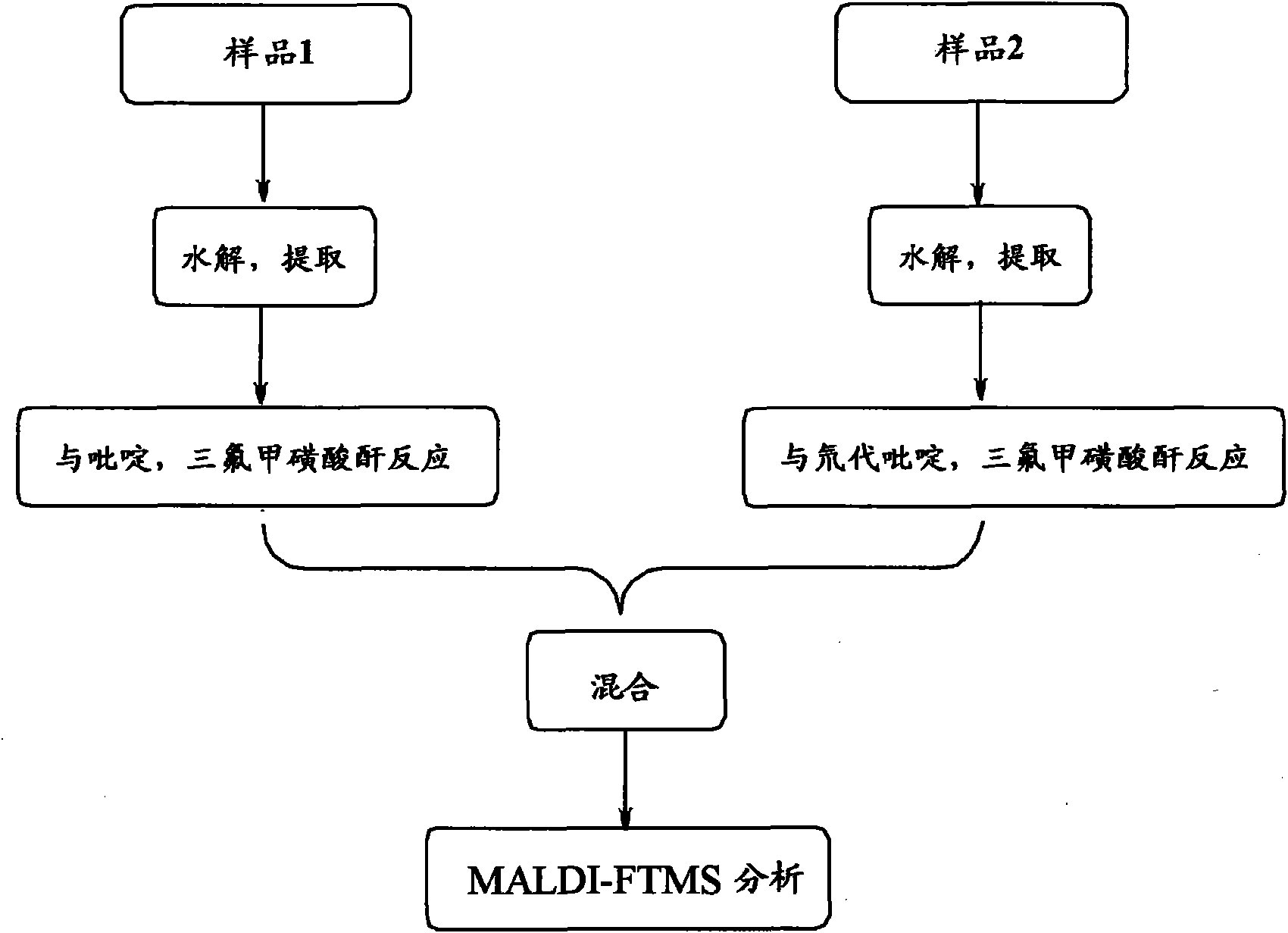
[0059] Example 1: Derivatization reaction and analysis of metabolites in urine samples
[0060] The operation steps are as follows:
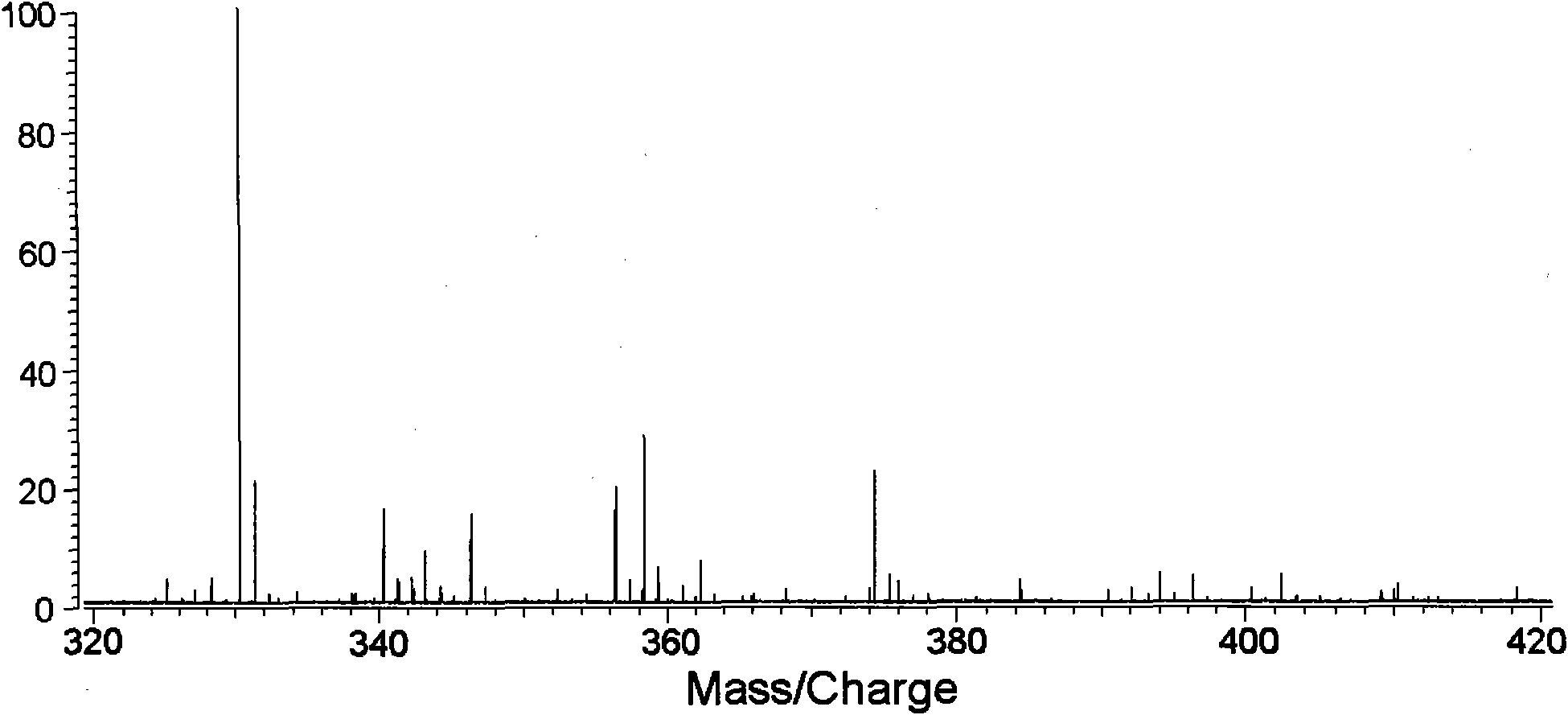
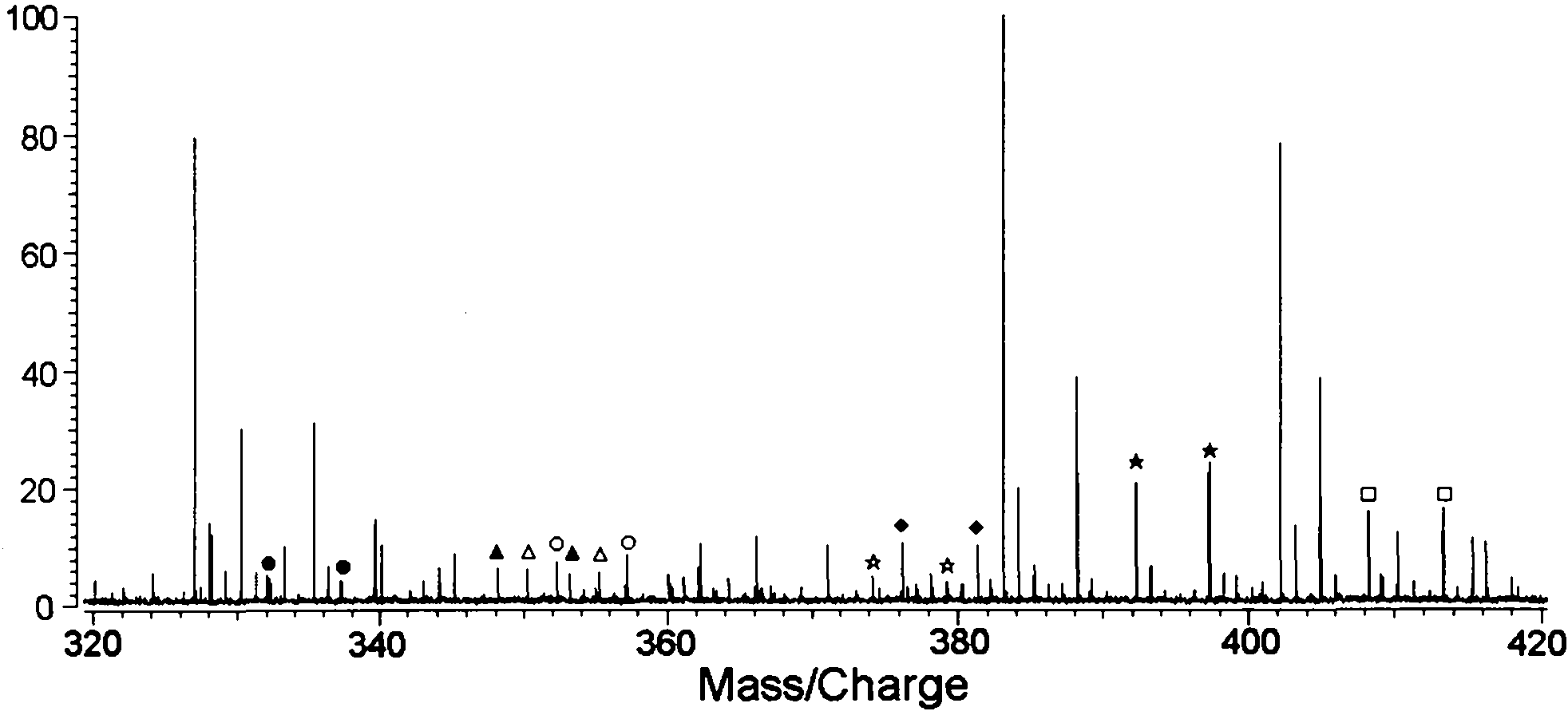
[0061] Divide 1-2mL urine sample into two equal parts, add 100-200μL 2M acetic acid-sodium acetate buffer (pH5.4-5.8) and 50-100μL β-glucosidase / sulfatase to each, and digest at 37-55℃ for 3h , add 2.5-5mL of tert-butyl methyl ether (TBME) to extract twice, combine the organic layers and blow dry with nitrogen, first add 50-100μL 10-40% pyridinedichloromethane solution, and then mix with 50-100μL 10 -40% trifluoromethanesulfonic anhydride dichloromethane mixed reaction for 5-20min, the second part was added 5-100μL 10-40% deuterated pyridine dichloromethane solution, and then mixed with 50-100μL 10-40% trifluoromethane Methanesulfonic anhydride and dichloromethane were mixed and reacted for 5-20 minutes. After mixing the two reaction solutions, they were mixed with 100-200 mg / mL matrix DHB acetone solution at a volume ratio of 1:3, and analyzed...
Embodiment 2
[0069] Embodiment 2: hair sample derivatization reaction and analysis
[0070] The operation steps are as follows:
[0071] Wash the hair with distilled water, dichloromethane and isopropanol, dry at 50-60°C, cut the hair into 1-2mm, weigh 0.3-0.4mg, add 200-500μL 6M sodium hydroxide and methanol (1:1), 60 Hydrolyze at -70°C for 1-2 hours, divide into two equal parts, add dichloromethane solution to extract twice, combine the organic layer and blow dry with nitrogen, add 50-100 μL 10-40% pyridine dichloromethane solution to the first part, and then Mix and react with 50-100 μL 10-40% trifluoromethanesulfonic anhydride dichloromethane for 5-20 minutes, add 50-100 μL 10-40% deuterated pyridine dichloromethane solution to the second part, and then mix with 50-100 μL 10- 40% trifluoromethanesulfonic anhydride and dichloromethane were mixed and reacted for 5-20 minutes. The two reaction solutions were mixed and then mixed with 100-200 mg / mL matrix DHB acetone solution at a volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com