Methylsulfonyl miazines isotope labelling reagent, synthesis method and uses thereof
An isotope labeling, sulfone-based pyrimidine technology, applied in biological testing, material testing products, measuring devices, etc., can solve the problems of quantitative error of labeling efficiency, high cost of isotope labeling reagents, etc., to achieve stable properties, improve reliability and accuracy Sex, the effect of reducing complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
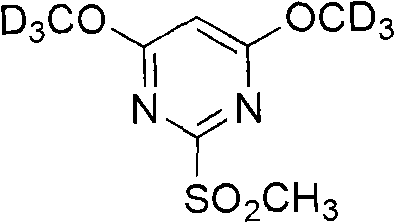
[0036] Example 1: [d 6 ]-4,6-dimethoxy-2-thiamphenicol pyrimidine ([d 6 ]-DMMSP) synthesis
[0037] The operation steps are as follows:
[0038] 4,6-Dichloro-2-methylthiopyrimidine (500mg, 2.5mmol) was dissolved in 10mL of anhydrous deuterated methanol, and gradually added dropwise to 20mL of deuterated sodium methoxide solution (200mg of sodium metal was dissolved in 20mL of anhydrous deuterated instead of methanol). After the reaction solution was stirred overnight at 25°C, it was concentrated by filtration, then dissolved in 10 mL of deionized water, and extracted with dichloromethane (3×5 mL). The organic phases were combined, dried over sodium sulfate, and spin-dried to obtain [d 6 ]-4,6-dimethoxy-2-methylthiopyrimidine as a crude product.
[0039] will [d 6 ] - The crude product (400 mg, 2.1 mmol) was dissolved in 40 mL of anhydrous dichloromethane, and 3-chloroperoxybenzoic acid (mCPBA, 1.4 g, 8 mmol) was added. After the mixture was stirred at 30 °C for 5 hours,...
Embodiment 2
[0043] Example 2: Labeling reaction of mixed polypeptides
[0044] The operation steps are as follows:
[0045] 1. Polypeptide mixture: His-Cys-Lys-Phe-Trp-Trp (HW-6), angiotensin I human acetate hydrate (DL-10), [Glu 1 1 μmol each of ]-fibrinopeptide B human (ER-14) and neurotensin (EL-13), dissolved in 500 μL of 0.03 M ammonium bicarbonate buffer solution (pH 8.0-8.2), and 2 μmol of dithiothreitol (DTT) was added , adjust the pH to 6.5 with acetic acid solution.
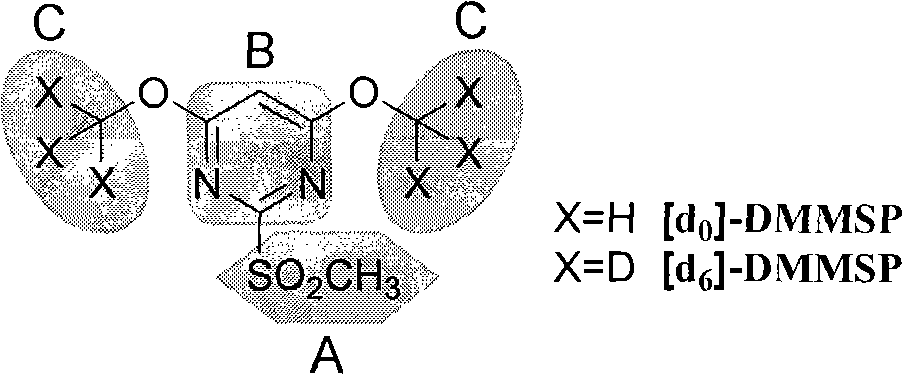
[0046] 2. Add [d 0 ]-DMMSP (5 μmol), the modification reaction was carried out in a constant temperature and humidity box at 37° C. for 2 hours.
[0047] 3. MALDI-TOF MS analysis conditions are: matrix α-cyano-4-hydroxycinnamic acid (α-CHCA), dissolved in 1:1 acetonitrile / water solution containing 0.1% trifluoroacetic acid, the concentration is 6mg / mL; Data acquisition was performed in positive ion, reflectance mode.
[0048] MALDI-TOF MS analysis results are as follows:
[0049] 1. [M+H] of only cysteine r...
Embodiment 3
[0051] Example 3: Comparative Analysis of Proteins
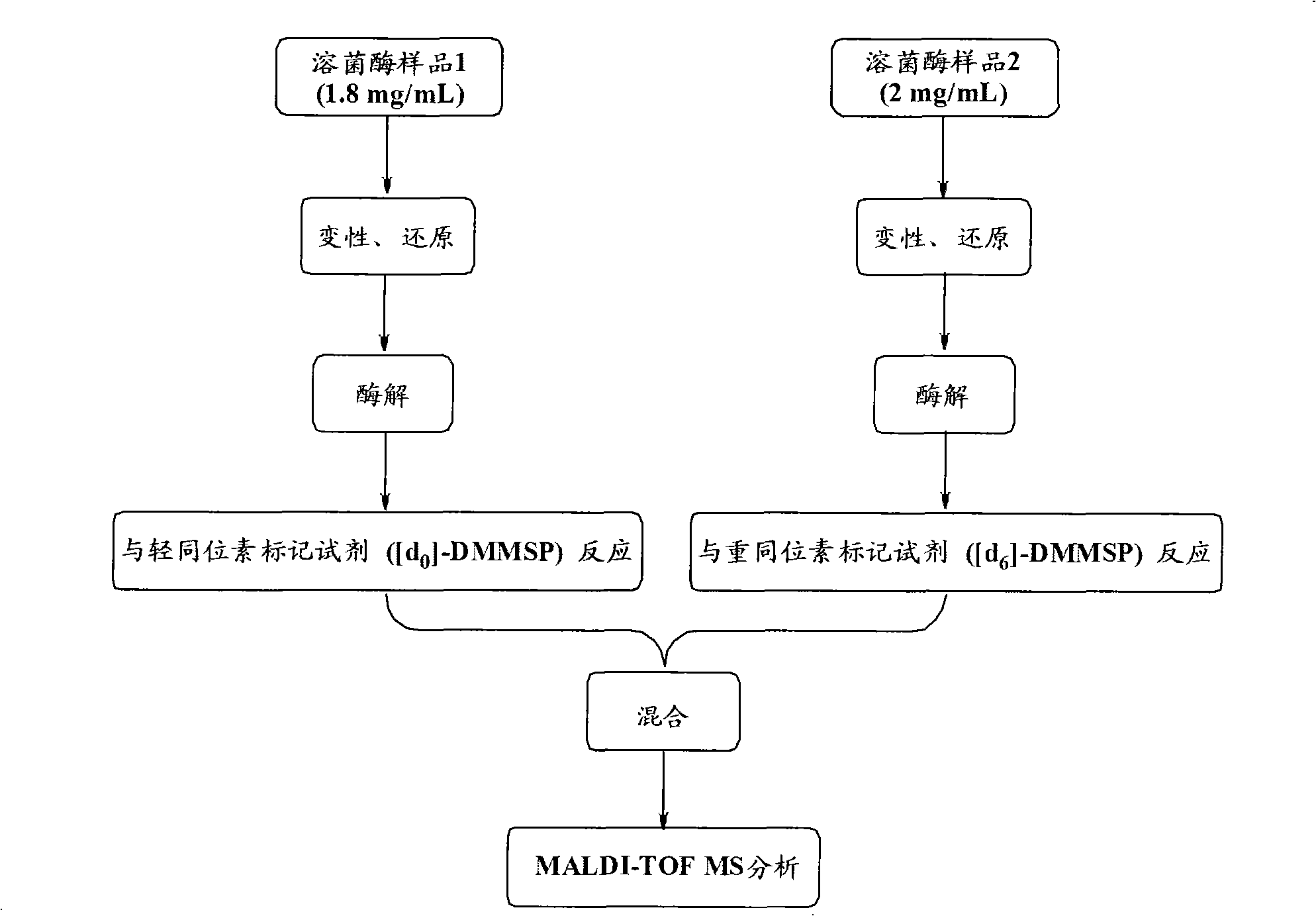
[0052] The experimental steps are attached figure 1 shown.
[0053] The operation steps are as follows:
[0054] 1. Two lysozyme standard products, 0.9mg and 1mg, were respectively dissolved in 500μL 30mmol / L ammonium bicarbonate buffer solution (pH 8.0-8.2, containing 8M urea), then respectively added 2μmol of DTT, and placed at 37℃ with constant temperature and humidity Keep warm in the box for 6 hours.
[0055] 2. After cooling, dilute the two lysozyme solutions with 30mmol / L ammonium bicarbonate buffer solution (pH 8.0-8.2) to 1mL respectively, and add trypsin according to the ratio of trypsin / lysozyme 1:50 (w / w) The solution was placed in a 37°C constant temperature and humidity box for enzymatic hydrolysis overnight.
[0056] 3. Dilute the two parts of lysozyme hydrolyzate to 2mL with 30mmol / L ammonium bicarbonate buffer solution (pH 8.0-8.2), adjust the pH to 6.5 with acetic acid solution, then add [d 0 ]-DMMSP (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com