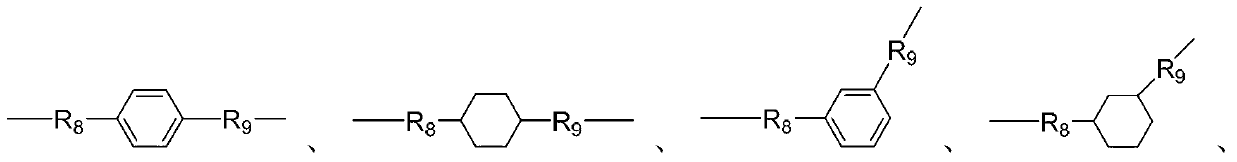
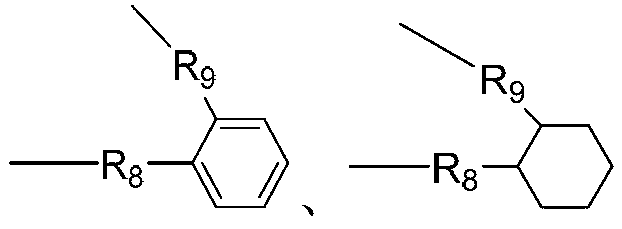
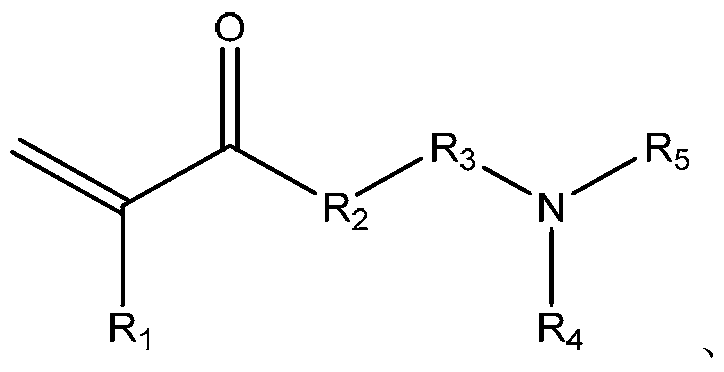
Synthesis method for betaine-type amphoteric ion compound containing reactive group
A zwitterion, synthesis method technology, applied in the preparation of organic compounds, chemical instruments and methods, cyanide reaction preparation and other directions, can solve problems such as inappropriate use, carcinogenicity, etc., and achieve the advantages of less side reactions, simple implementation, and reduced synthesis costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Add 119.16g (1.0mol) of N-methyldiethanolamine and 100g of acetone into the flask, and dropwise add 144.12g (2.0mol) of acrylic acid and 150g of acetone solution while stirring. The dropwise addition is completed within 0.5 hours, and the temperature rises to 45°C and react for 1 hour. The reaction system was lowered to room temperature, filtered and washed 3 times with acetone to obtain a white solid A with a yield of 87.6%. The structure of product A is shown as follows by infrared and hydrogen nuclear magnetic resonance analysis. The proton nuclear magnetic resonance spectrum of product A is as attached figure 1 shown. The carbon magnetic resonance spectrum of product A is attached figure 2 shown.
[0050]
[0051] Among them, attached drawing l 1 In the HNMR spectrum, the characteristic peak with a chemical shift of 3.16ppm is the proton peak at a; the characteristic peak with a chemical shift of 3.55-3.58ppm is the proton peak at b; the characteristic peak...
Embodiment 2
[0055] Add 156.2g (1mol) of N,N'-dimethylaminopropylacrylamide and 150g of acetonitrile into the flask, add 14.41g (0.2mol) of acrylic acid dropwise while stirring, and add dropwise within 0.5 hours After completion, the temperature was raised to 50° C., and the reaction was carried out for 120 hours. The reaction system was lowered to room temperature, filtered and washed 3 times with acetonitrile to obtain white solid B with a yield of 86.2%. The structure of the product B is shown as follows by infrared and hydrogen nuclear magnetic resonance analysis. The proton nuclear magnetic resonance spectrum of product B is as attached image 3 shown. The carbon magnetic resonance spectrum of product A is attached Figure 4 shown.
[0056]
[0057] Among them, attached image 3 1 In the HNMR spectrum, the characteristic peaks with a chemical shift of 5.75-6.28ppm are the proton peaks at a and b; the characteristic peaks with a chemical shift of 3.29-3.37ppm are the proton pe...
Embodiment 3
[0059] Add 89.14g (1.0mol) of N,N'-dimethylethanolamine and 100g of tetrahydrofuran into the flask, and add 64.05g (0.5mol) of 4-carbonyl-5-hexenoic acid and 70g of The tetrahydrofuran solution was added dropwise within 0.5 hours, and reacted at 0°C for 120 hours. The reaction system was cooled to room temperature, filtered and washed 3 times with chloroform to obtain white solid C with a yield of 90.1%. Through infrared and proton magnetic resonance analysis identical with embodiment 1, 2, the structure of determining product C easily is as follows:
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com