Chiral fluoroquinolone C-3 fused heterocycle alpha, beta-unsaturated ketone derivative as well as preparation method and application thereof
A fluoroquinolone, C-3 technology, applied in the field of chiral fluoroquinolone C-3 fused heterocycle α, to achieve the effect of increasing anti-tumor activity and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
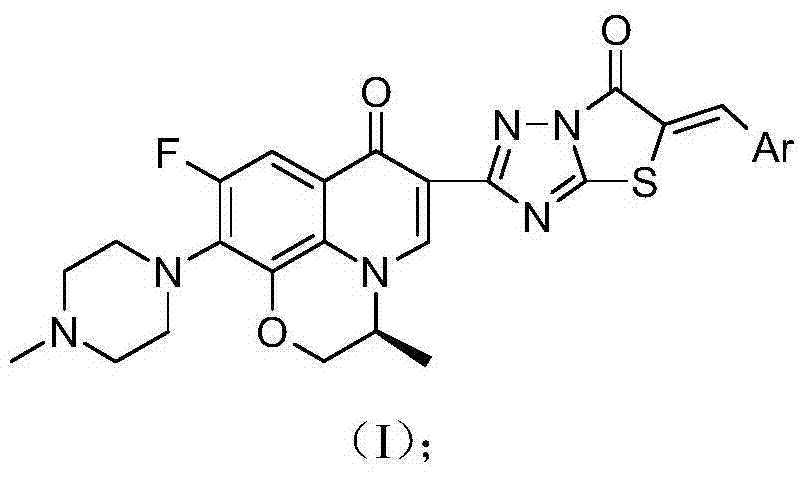
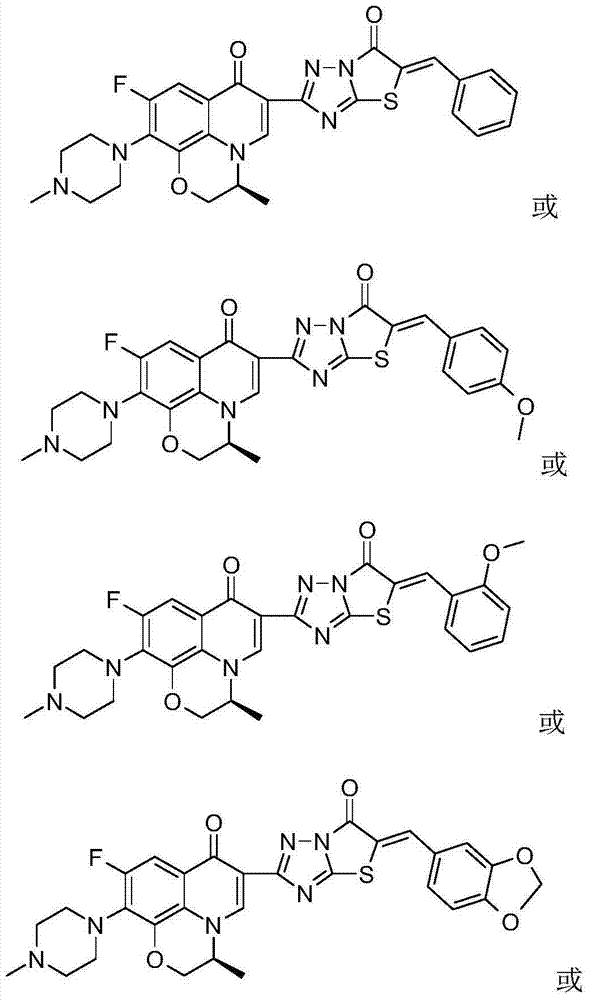
[0055] The chiral fluoroquinolone C-3 condensed heterocyclic α, β-unsaturated ketone derivative in this example is (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1, 8-(3,1-Oxopropyl)-3-[5-Benzylidene-thiazolo[3,2-b][1,2,4]triazol-6(5H)-one-2- Base]-quinolin-4(1H)-one, its chemical structural formula is:
[0056]
[0057] That is, Ar in formula (I) is phenyl.
[0058] The preparation method of the chiral fluoroquinolone C-3 condensed heterocyclic α, β-unsaturated ketone derivative of the present embodiment is as follows: take 1.0 g (2.4 mmol) of (S)-6-fluoro-7-(4-methyl Base-piperazin-1-yl)-1,8-(3,1-oxopropyl)-3-(5-mercapto-1,2,4-4H(1H)triazol-3-yl)-quinone Lin-4 (1H)-one (V) and 0.31g (2.9mmol) of benzaldehyde, 0.27g (2.9mmol) of chloroacetic acid, add glacial acetic acid (7ml) containing 0.24g (2.9mmol) of anhydrous sodium acetate In a mixed solvent with acetic anhydride (3ml), reflux reaction for 12h; evaporate the solvent under reduced pressure, add absolute ethanol (10ml) ...
Embodiment 2
[0060] The chiral fluoroquinolone C-3 condensed heterocyclic α, β-unsaturated ketone derivative in this example is (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1, 8-(3,1-Oxopropyl)-3-[5-p-methoxybenzyl-thiazolo[3,2-b][1,2,4]triazol-6(5H)-one -2-yl]-quinolin-4 (1H)-one, its chemical structural formula is:
[0061]
[0062] That is, Ar in formula (I) is p-methoxyphenyl.
[0063] The preparation method of the chiral fluoroquinolone C-3 condensed heterocyclic α, β-unsaturated ketone derivative of the present embodiment is as follows: take 1.0 g (2.4 mmol) of (S)-6-fluoro-7-(4-methyl Base-piperazin-1-yl)-1,8-(3,1-oxopropyl)-3-(5-mercapto-1,2,4-4H(1H)triazol-3-yl)-quinone Lin-4(1H)-ketone (V) and 0.40g (2.9mmol) of p-methoxybenzaldehyde, 0.21g (2.5mmol) of chloroacetic acid, add ice containing 0.24g (2.9mmol) of anhydrous sodium acetate In a mixed solvent of acetic acid (7ml) and acetic anhydride (3ml), reflux for 12 hours; evaporate the solvent under reduced pressure, add absolut...
Embodiment 3
[0065] The chiral fluoroquinolone C-3 condensed heterocyclic α, β-unsaturated ketone derivative in this example is (S)-6-fluoro-7-(4-methyl-piperazin-1-yl)-1, 8-(3,1-Oxopropyl)-3-[5-o-methoxybenzyl-thiazolo[3,2-b][1,2,4]triazol-6(5H)-one -2-yl]-quinolin-4 (1H)-one, its chemical structural formula is:
[0066]
[0067] That is, Ar in formula (I) is o-methoxyphenyl.
[0068] The preparation method of the chiral fluoroquinolone C-3 condensed heterocyclic α, β-unsaturated ketone derivative of the present embodiment is as follows: take 1.0 g (2.4 mmol) of (S)-6-fluoro-7-(4-methyl Base-piperazin-1-yl)-1,8-(3,1-oxopropyl)-3-(5-mercapto-1,2,4-4H(1H)triazol-3-yl)-quinone Lin-4(1H)-one (V) and 0.40g (2.9mmol) of o-methoxybenzaldehyde, 0.23g (2.5mmol) of chloroacetic acid, add ice containing 0.24g (2.9mmol) of anhydrous sodium acetate In a mixed solvent of acetic acid (7ml) and acetic anhydride (3ml), reflux for 12 hours; evaporate the solvent under reduced pressure, add absolute e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com