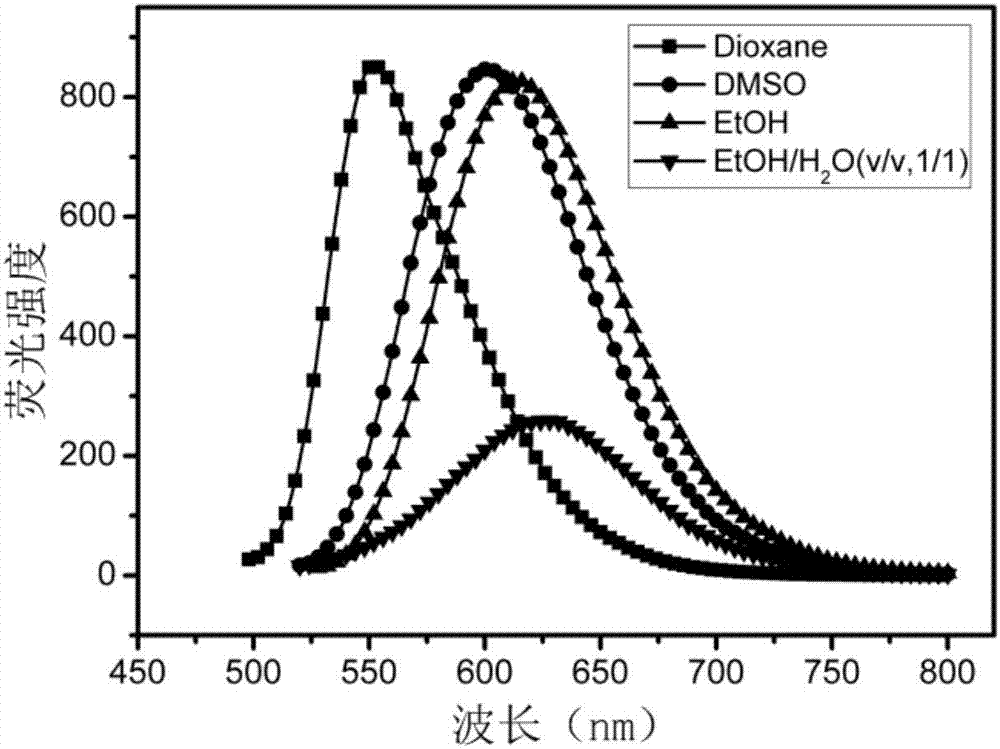
Lysosomal targeting fluorescent probe and preparation method thereof
A fluorescent probe and lysosome technology, applied in fluorescence/phosphorescence, carboxylic acid amide preparation, cyanide reaction preparation, etc., can solve the problems of large background signal interference, high price, and few types, and achieve low background interference , reduce interference, and sensitive method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
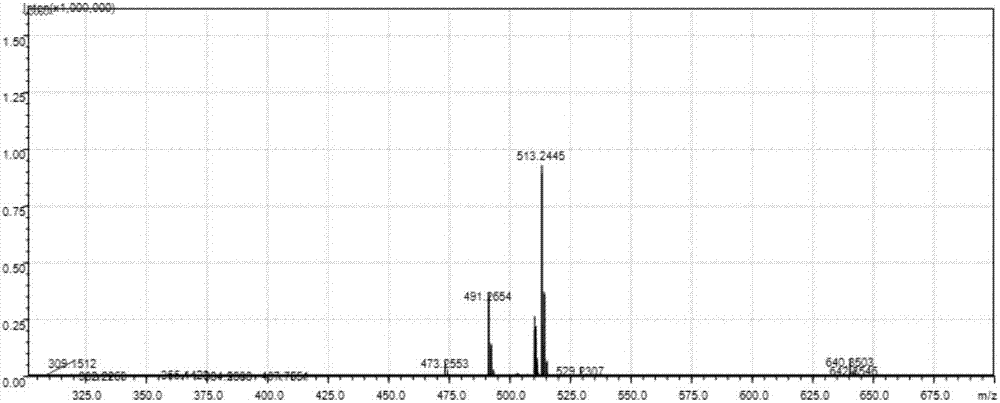
[0036] Example 1: Synthesis of Formula I
[0037]
[0038] (1) Synthesis of intermediate M1: under argon protection, compound 3-(tert-butylacetyl)acetylacetone (1.40g, 6.53mmol), diboron trioxide (0.46g, 6.61mmol) and tributyl borate (3.01 g, 13.07 mmol) was dissolved in 5 mL of DMF (solvent I), heated to 75 °C and stirred for 15 minutes, then 4-dimethylaminobenzaldehyde (2.05 g, 13.74 mmol) and piperidine (40 μL) (catalyst I) were added in sequence ), heated to 90°C (reaction temperature I) and reacted for 4 hours (reaction time I). After the reaction was completed, it was cooled to 70° C., 50 mL of 20% aqueous acetic acid solution was added, and stirring was continued for 1 hour. After cooling to room temperature, 100 mL of chloroform was added for extraction, the organic phase was washed three times with water, dried by adding anhydrous sodium sulfate, concentrated and separated with a silica gel column. The eluent was chloroform / ethanol (100:1) to obtain compound M1 (2...
Embodiment 8
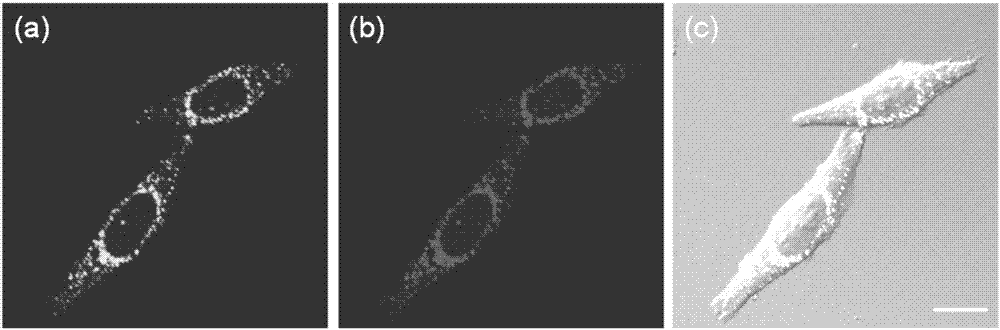
[0043] Example 8, lysosome colocalization experiment of formula I
[0044]In order to investigate the ability of formula I to localize to lysosomes in cells, we performed lysosome co-localization experiments on formula I. HeLa cells were stained with LysoSensor Green DND-189 (1 μM, a commercial lysosomal stain) and Formula I (5 μM), respectively, as shown in the appendix. image 3 shown. from image 3 Green punctate fluorescence from LysoSensor Green DND-189 can be observed in a. from image 3 In b, bright punctate red fluorescence can be observed in the perinuclear region of HeLa cells. Will image 3 a and 3b are superimposed to get image 3 c, It was found that the coincidence of the two was as high as 95%, which proved that the probe formula I could selectively localize to the lysosome and emit strong red fluorescence.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com