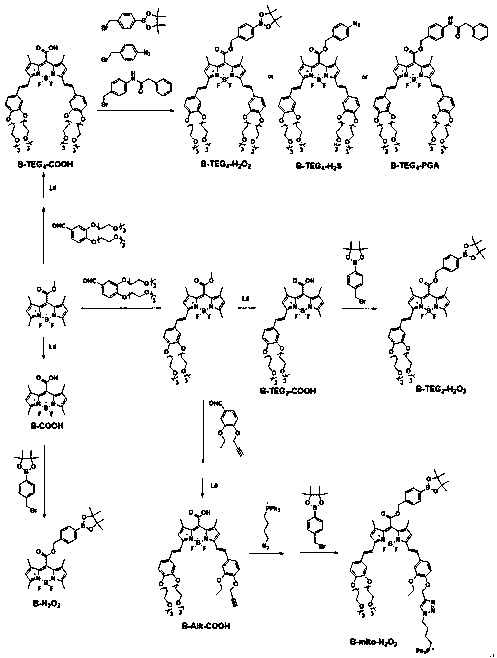
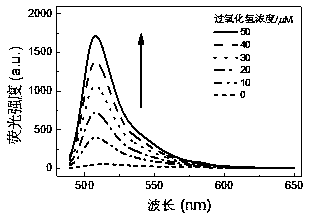
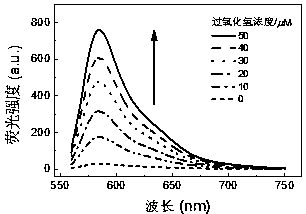
BODIPY-based high-sensitivity fluorescent probe and synthesis method and application thereof
A technology of fluorescent probe and synthesis method, which is used in biological analysis and imaging applications, and the synthesis of high-sensitivity fluorescent probes, which can solve the problems of insufficient cell membrane permeability and poor water solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The synthesis route of BODIPY mother core is as follows:
[0036]
[0037] The synthesis method is as follows:
[0038] Under an ice-water bath, methanol (1.60 g, 50.0 mmol) was slowly added dropwise to oxalyl chloride (6.35 g, 50.0 mmol) in dichloromethane (CH 2 Cl 2 , 100 mL) solution, then stirred at room temperature for 30 min, and the solvent was removed by rotary evaporation, and the obtained methyl oxalyl chloride was directly used in the next reaction. Dissolve 2,4-dimethylpyrrole (7.14g, 75.0mmol) in dichloromethane (140mL), cool to -78°C, slowly add methyl oxalyl chloride (3.67g, 30.0mmol) in dichloromethane (80mL) solution, then continued stirring at -78°C for 2h, and slowly added triethylamine (Et 3 N, 12.14g, 120.0mmol), boron trifluoride ether (BF 3 ·Et 2 (0, 22 mL), raised to room temperature, and continued to stir for 2h. The solvent was removed by rotary evaporation, and B-Me (3.67 g, yield 40.0%) was isolated by passing through the column with...
Embodiment 2
[0040] The synthetic routes of Alk-Ba and Azide-tpp are as follows:
[0041]
[0042] The synthesis method is as follows:
[0043] Stir 3,4-dihydroxybenzaldehyde (2.76g, 20.0mmol), potassium carbonate (2.76g, 20.0mmol) in acetone (30mL) for 10min, and slowly add propargyl bromide (2.38g, 20.0mmol) dropwise at room temperature ) in acetone (50 mL) for two hours and stirred for 14 hours. Filtrate, spin evaporate the filtrate to remove acetone, dissolve with ethyl acetate (100mL), wash with distilled water (100mL) and saturated sodium chloride solution (100mL) successively once, the organic layer is dried with anhydrous sodium sulfate, spin evaporate to remove solvent, use The dichloromethane:petroleum ether mixed solvent with a volume ratio of 1:1 was passed through the column as an eluent, and 3-hydroxy-4-propargyloxybenzaldehyde (1.73 g, yield 49.1%) was isolated.
[0044] 3-Hydroxy-4-propargyloxybenzaldehyde (1.70g, 10.0mmol), potassium carbonate (2.76g, 20.0mmol) was st...
Embodiment 3
[0048] B-TEG 4 The synthetic route of -COOH is as follows:
[0049]
[0050] The synthesis method is as follows:
[0051] At 0°C, a solution of p-toluenesulfonyl chloride (20.97g, 110.0mmol) in tetrahydrofuran (100mL) was slowly added dropwise to triethylene glycol monomethyl ether (16.42g, 100.0mmol) and triethylamine (22.26g, 220.0mmol) ) in dichloromethane (300mL) solution, then stirred at room temperature for 14 hours, filtered, the filtrate was washed with distilled water (150mL; 1 time), saturated ammonium chloride solution (150mL×3 times), and the organic layer was washed with anhydrous sulfuric acid After drying over sodium, the solvent was removed by rotary evaporation, and the column was separated with petroleum ether and ethyl acetate (V:V=10:1) as eluents to obtain Ts-TEG (30.88 g, yield 97.0%).
[0052] 3,4-dihydroxybenzaldehyde (1.38g, 10.0mmol), potassium carbonate (K 2 CO 3 , 6.91g, 50.0mmol), potassium iodide (KI, 0.30g) were stirred in acetone (200mL) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com