Near infrared BODIPY (Boron Dipyrromethene Compounds) hydroxyl radical probes and synthesis method and usage thereof
A near-infrared and compound technology, which is applied in chemical instruments and methods, compounds of group 5/15 elements of the periodic table, material inspection products, etc., to achieve rapid and efficient detection of hydroxyl radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Synthesis of Compounds B1-B4:
[0031]
[0032] In a 250 mL round bottom flask, add (2 mmol) corresponding aldehyde, (4 mmol) 2,4-dimethylpyrrole, 0.01 mL of trifluoroacetic acid and 100 mL of anhydrous dichloromethane, and stir overnight at room temperature. Then add (2 mmol) DDQ for oxidation, react for 1 h, add 3 mL of triethylamine and boron trifluoride ether solution in turn, react for 5 h, add water to quench the reaction. After the reaction is finished, extract with dichloromethane, spin dry under reduced pressure, perform column chromatography, and use dichloromethane to petroleum ether as a developer of 1:2 to pass through the column to obtain the corresponding BODIPY product.
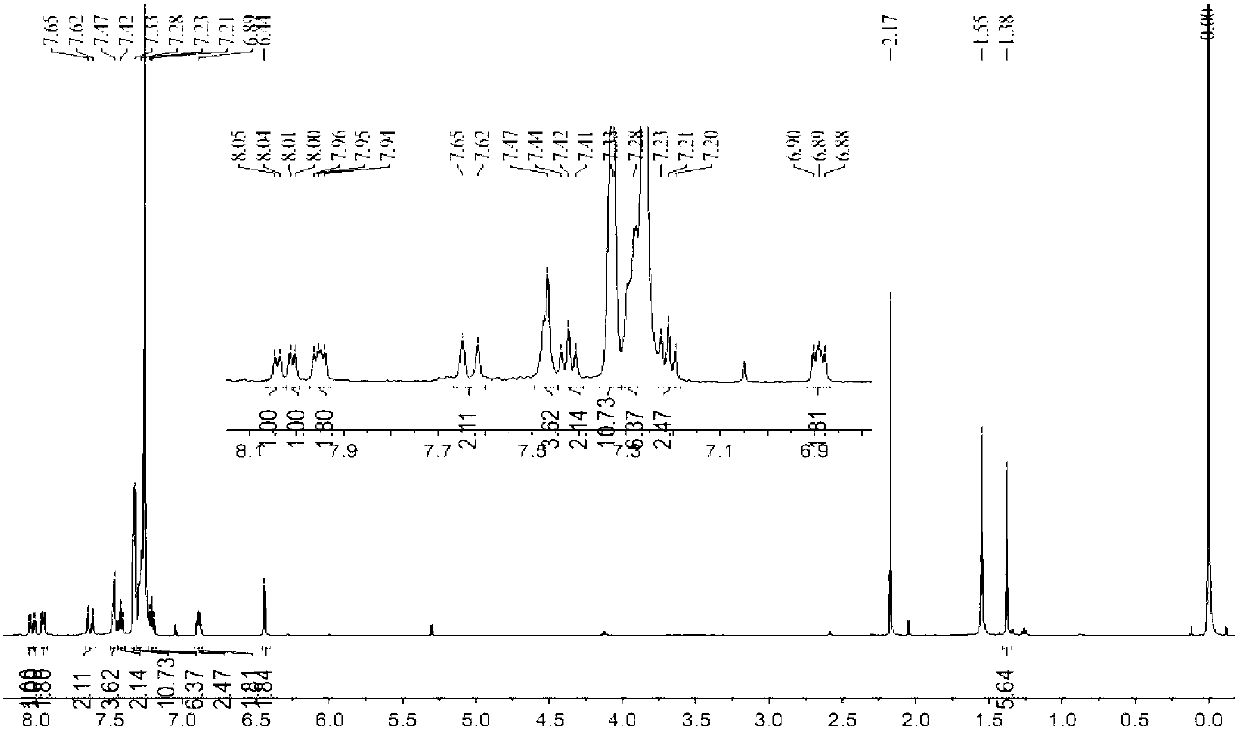
[0033] B1C 19 h 19 BF 2 N 2 (324.16) yellow solid, 38.6% yield, 1 H NMR (CDCl 3 , 500MHz): 7.45(t, J=5Hz, 3H), 7.27(s, 2H), 6.95(s, 2H), 2.53(s, 6H), 1.34(s, 6H).
[0034] B2C 51 h 83 BF 2 N 2 o 2 (804.65) red solid, 62.2% yield, 1 H NMR (CDCl 3 , 500MHz): 6....
Embodiment 2
[0037] Example 2. Synthesis of Compounds B5-B8:
[0038]
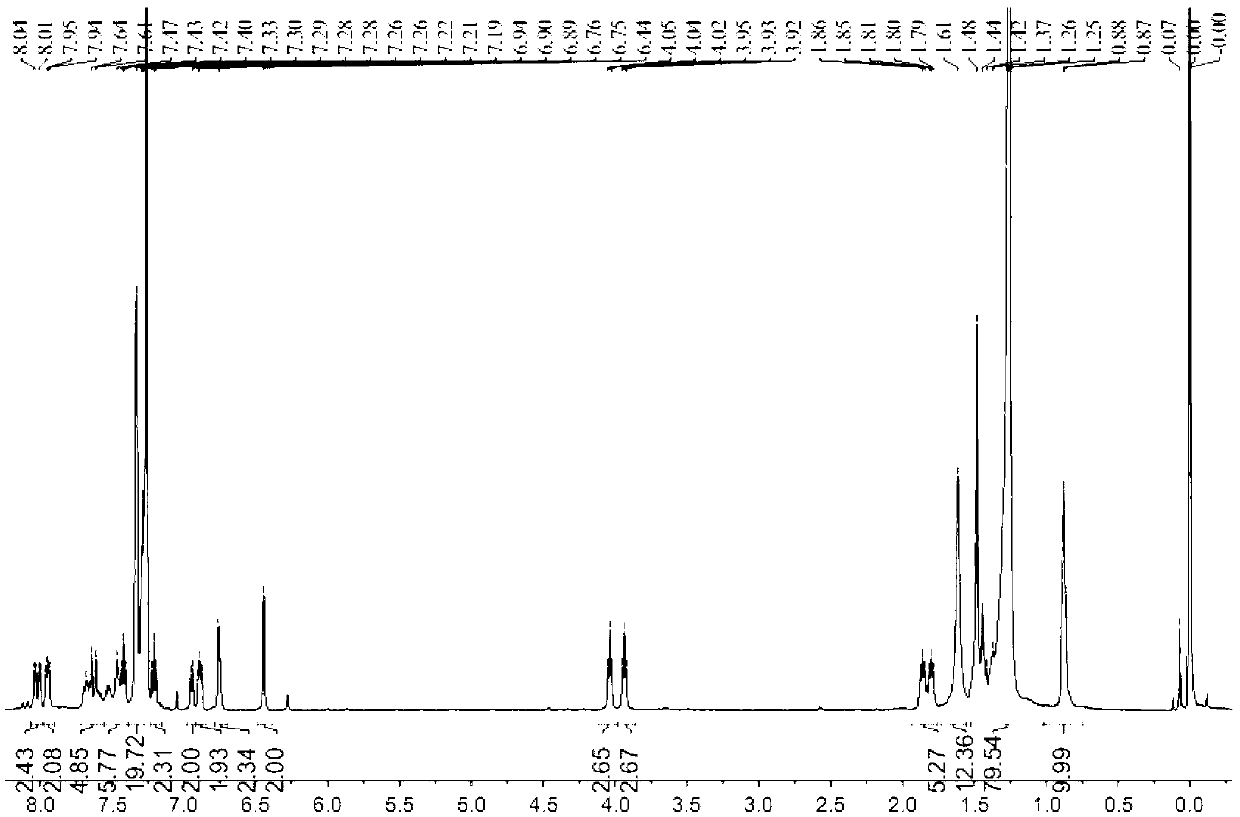
[0039] In a 100mL two-necked flask, add (0.16mmol) simple BODIPY compounds B1-B4, (0.32mmol) 2-(diphenylphosphine)benzaldehyde, 0.4mL anhydrous piperidine, 0.4mL glacial acetic acid and 50mL anhydrous Water acetonitrile, under the protection of argon, using a water separator, heated to 90 ° C, the reaction process was monitored by TLC, until all the raw material points B1-B4 disappeared. After the reaction was completed, it was cooled to room temperature. After the reaction, a large amount of solids were precipitated, which were directly sucked and filtered to obtain a solid as the product. The filtrate was extracted with water and saturated brine respectively, dried over anhydrous sodium sulfate, and the crude product was obtained under reduced pressure. The target compounds B5-B8 were obtained by column chromatography. NMR spectrum see attached Figure 1-Figure 4 .
Embodiment 3
[0040] Example 3. Synthesis of Compounds B9 and B10:
[0041]
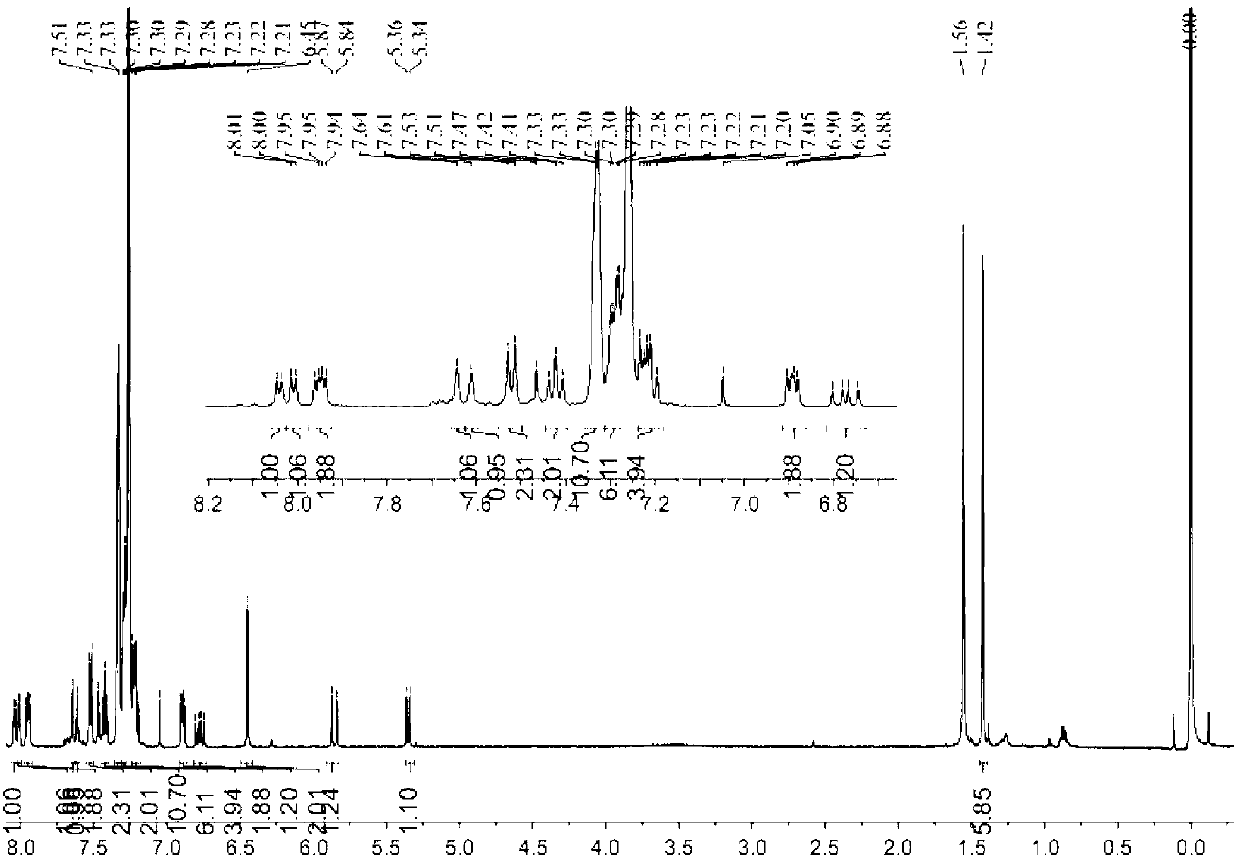
[0042] In a 100mL round bottom flask, add (0.02mmol) compound B5 or B6, dissolve it in tetrahydrofuran, then add (0.2mmol) hydrogen peroxide, then add (0.12mmol) cobalt acetate, react for 30 minutes, and the reaction process is analyzed by TLC Monitor until all raw material points disappear. The filtrate was extracted with water and saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain the pure product. NMR spectrum see attached Figure 5 and Figure 6 . The crystal structure of probe B9 is attached Figure 7 , the test data of probe B9 single crystal is shown in Table 1.
[0043] Table 1 Single crystal data of probe B9
[0044]
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com