Near infrared BODIPY fluorescence dye and preparation method thereof
A fluorescent dye and near-infrared technology, which is applied in the field of organic compound synthesis, can solve the problems of many synthesis steps, poor solubility, and high difficulty, and achieve the effects of good solubility, less pollution, and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
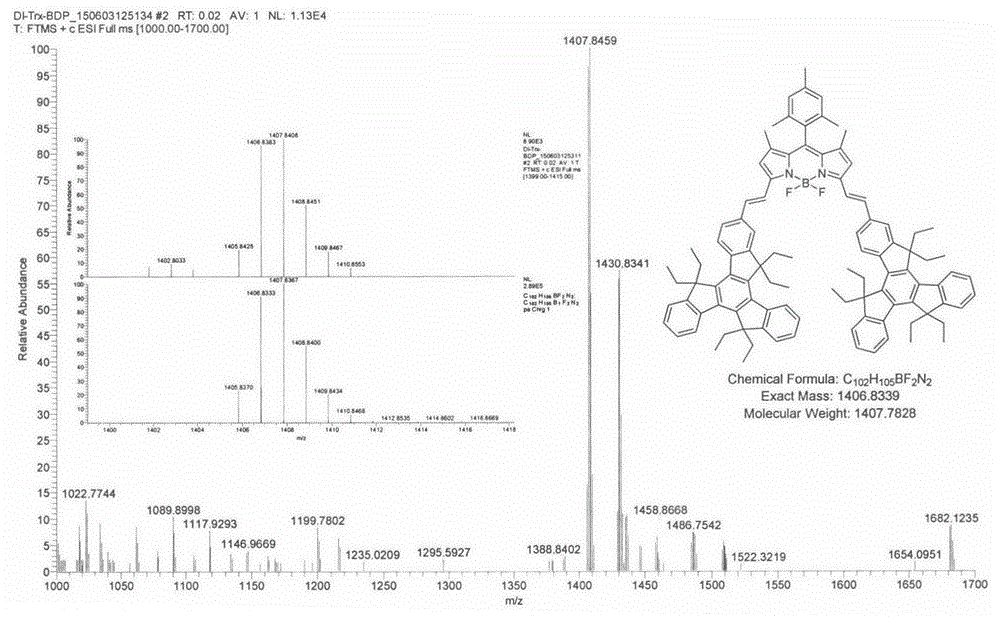
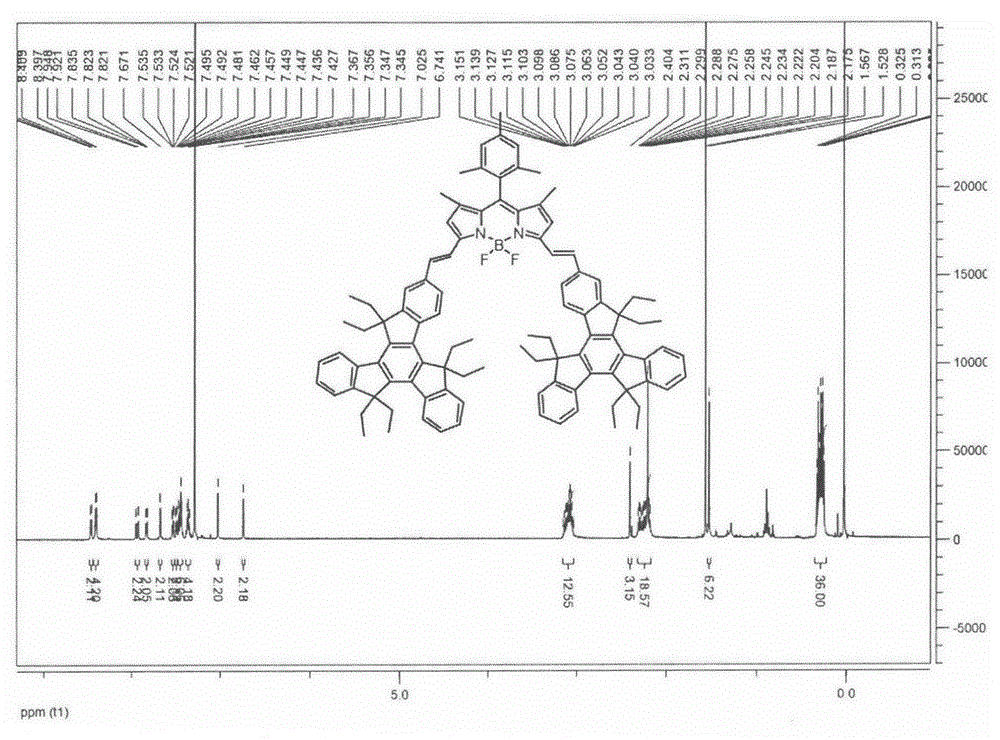
[0032] Equipped with a water trap in the single-neck round bottom flask, 1,3,5,7-tetramethyl-8(2,4,6-trimethylbenzene)-BODIPY (146.4mg, 0.40mmol), 2-formyl Indene (0.862g, 1.60mmol) and p-toluenesulfonic acid (68mg) were dissolved in 25mL toluene and 2mL piperidine, the mixture was heated to reflux at 140°C, TLC was followed to detect the complete reaction of the raw materials, and the solvent was collected until evaporated to dryness. The reactant was concentrated and subjected to silica gel column chromatography, and the eluent was (petroleum ether / CH 2 Cl 2 =6:4), to obtain dark green solid A (182.0 mg, 32.33%). Esi-MS: calcdforC 102 h 106 BF 2 N 2 1407.8417, found: 1407.8459 (M+H + )( figure 1 ); 1 HNMR: (600MHz, CDCl 3 )δ8.46(d, J=4.20Hz, 2H), 8.40(d, J=7.20Hz, 4H), 7.93(d, J=16.2Hz, 2H), 7.83(d, J=8.40Hz, 2H) , 7.67(s, 1H), 7.54-7.52(m, 2H), 7.50-7.48(m, 2H), 7.46-7.42(m, 6H), 7.38-7.33(m, 4H), 7.03(s, 2H) , 6.74(s, 2H), 3.15-3.03(m, 12H), 2.40(s, 3H), 2.31-2....
Embodiment 2
[0034] Equipped with a water trap in the round bottom flask, 1,3,5,7-tetramethyl-2,6-diiodo-8(2,4,6-trimethylbenzene)-BODIPY (247.2mg, 0.40mmol) , tripolyindene formaldehyde (0.862g, 1.60mmol) and p-toluenesulfonic acid (68mg) were dissolved in 25mL toluene and 2mL piperidine, the mixture was heated to reflux at 140 ° C, TLC traced and detected that the reaction of the raw materials was complete, and the solvent was collected until evaporated to dryness. The reactant was concentrated and subjected to silica gel column chromatography, and the eluent was (petroleum ether / CH 2 Cl 2 =8:2), to obtain green solid B (196.2mg, 29.54%). Esi-MS: calcdforC 102 h 103 BF 2 I 2 N 2 1658.6272, found: 1658.6218 (M + )( Figure 5 ); 1 HNMR: (600MHz, CDCl 3 )δ8.46(d, J=8.40Hz, 2H), 8.39(t, J=8.40Hz, 6H), 7.95(d, J=16.2Hz, 2H), 7.88(d, J=7.20Hz, 2H) , 7.69(s, 2H), 7.49-7.33(m, 12H), 7.06(s, 2H), 3.14-2.99(m, 12H), 2.43(s, 3H), 2.33-2.13(m, 18H), 1.56 (s, 6H), 0.34-0.23 (m, 36H) ( F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com