Fluorescent probe for detecting cyanide ion and synthesis and application method thereof
A fluorescent probe, cyanide ion technology, applied in the field of chemical analysis and detection, can solve the problems of insufficient sensitivity, high cost, insufficient probe selectivity, etc., and achieves a simple synthesis method, fast response speed, and good application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] [Example 1] Synthesis of fluorescent probe HB molecules that detect cyanide ions
[0039] The present invention detects the synthesis of the fluorescent probe molecule of cyanide ion, is to take 2,4-dimethylpyrrole and 4-hydroxymethyl benzaldehyde as raw material, through condensation, oxidation, then with 1,2,3,3- Tetramethyl-3H-indole iodide salt is obtained by Ke Naowen Geier condensation reaction.
[0040] (1) Synthesis of 1,3,5,6-tetramethyl 8-(4(hydroxymethylphenyl)) boron fluoride dipyrrole (2):
[0041] Take a dry 500ml two-necked bottle, replace the bottle with nitrogen, add 2,4-dimethylpyrrole (2.2mL, 20.0mmol), benzaldehyde (1.4g, 10.0mmol), trifluoroacetic acid (0.11mL, 1mmol) and CH 2 Cl 2 (150mL), stirred at room temperature for 12h, then added 2,3-dichloro-5,6-dicyano-p-benzoquinone (2.3g, 10.0mmol), stirred at room temperature for 1h, added Et 3 N (30mL), cooled to 0°C in an ice bath, added BF 3 .Et 2 O (30mL), stirred at room temperature for 3h, a...
Embodiment 2
[0046] [Example 2] The recognition performance of the fluorescent probe HB for detecting cyanide ions to anions
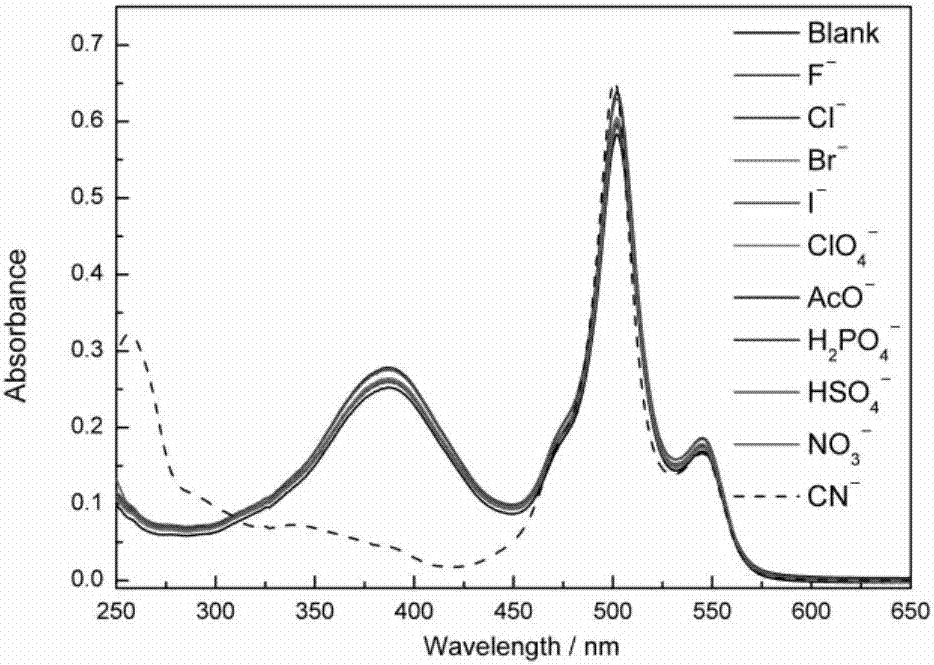
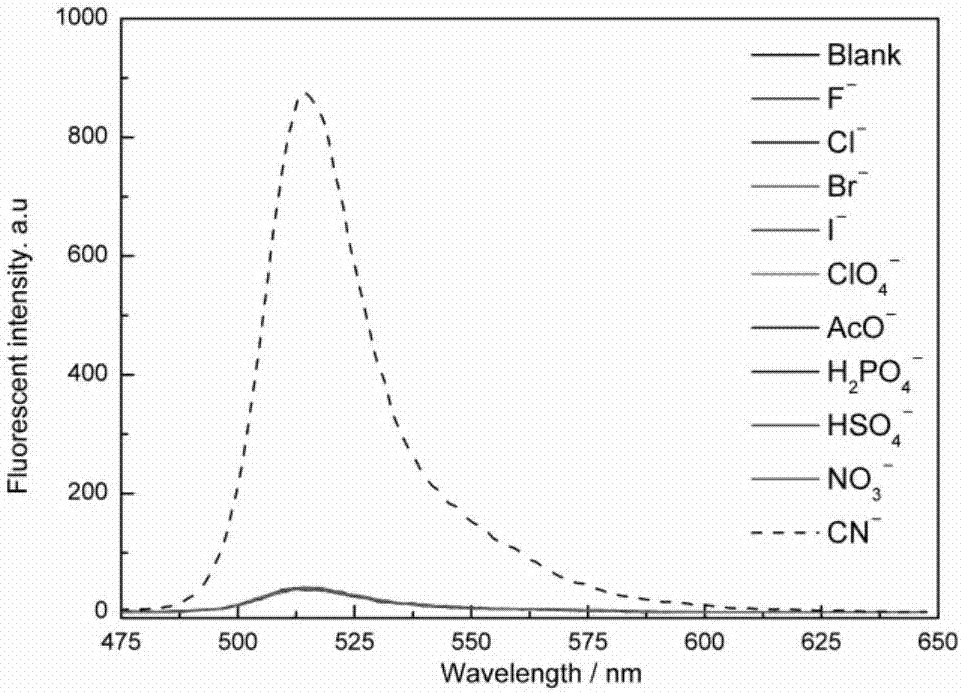
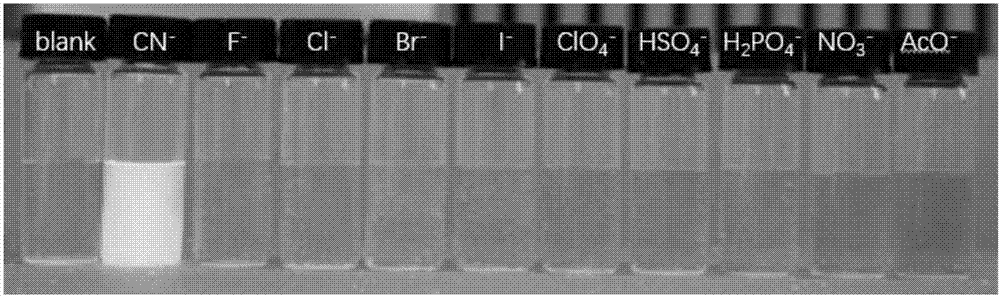
[0047] 1. Study on the selectivity of fluorescent probes for cyanide ions
[0048] Dissolve HB in absolute ethanol to prepare 10 -3 mol L -1 stock solution; prepare F separately ─ , Cl ─ 、Br ─ , I ─ , NO 3 ─ , ClO 4 ─ 、HSO 4 ─ 、H 2 PO 4 ─ 、AcO ─ 、CN ─ Aqueous solution of 10 -2 mol L -1 . Add 25 μL of HB stock solution and 2475 μL of water and ethanol (V / V=1:1) mixed solution to the cuvette, detect the ultraviolet absorption spectrum and fluorescence emission spectrum, add 20 μL of ion stock solution respectively, the concentration of HB at this time 10×10 -6 mol L -1 , the concentration of the anion is 8 times that of the acceptor, and the detection ultraviolet absorption spectrum and fluorescence emission spectrum (λ ex =383nm), observe the response of fluorescent probe HB to various anions.
[0049] The results show that HB has absorption p...
Embodiment 3
[0064] [Example 3] HB is used for the detection of actual water samples
[0065] Take 50mL of tap water, lake water and mineral water respectively in beakers, put them into sample tubes and centrifuge them with a centrifuge, and collect the supernatant after centrifugation. Add 25 μL of HB stock solution and 2475 μL (tap water / ethanol=1 / 1) mixed solution into the cuvette. Shake and check the fluorescence emission spectrum. Add 5 μmol·L to the cuvette -1 CN - , shake, detect the fluorescence emission spectrum, repeat this operation three times. Repeat the above operation for each water sample.
[0066] The results showed that the concentrations of cyanide ions in all three water samples were lower than 1.9 μmol L -1 , add 5 μmol·L -1 CN - After that, CN was measured in tap water, lake water and mineral water - The concentration is 5.27±0.19μmol L -1 , 5.23±0.11 μmol L -1 , 5.13±0.18 μmol L -1 . The recoveries of standard addition were 105.46±3.67%, 104.05±2.03%, 102...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com