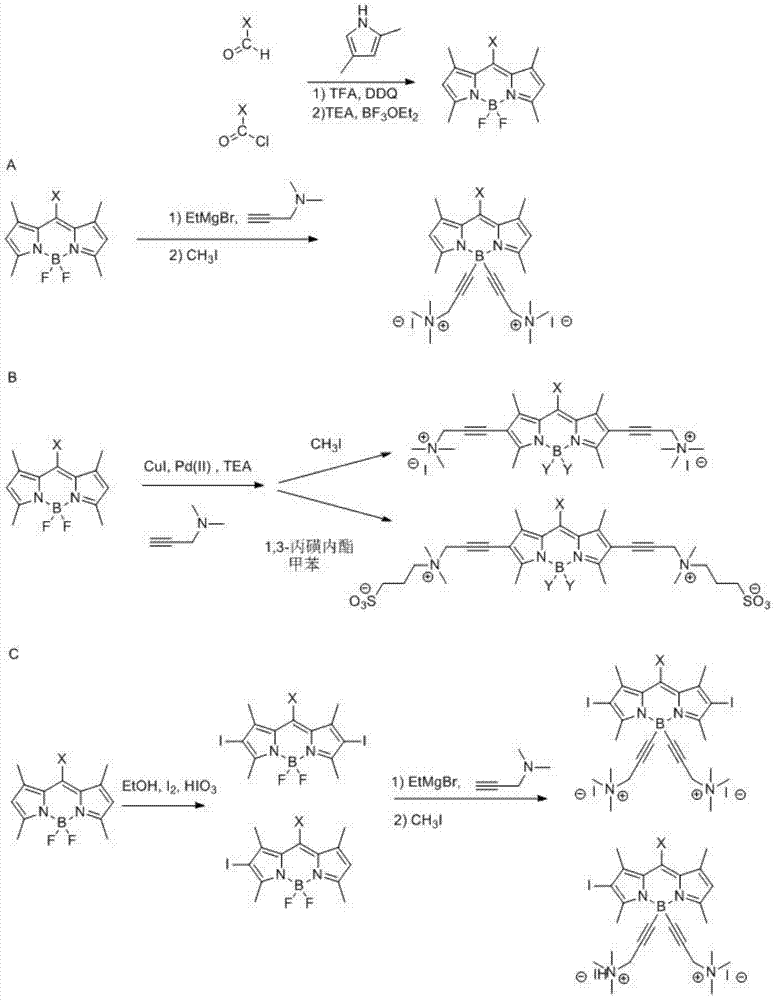
Boron fluoride dipyrrole fluorescent dye containing hydrophilic groups and preparation method thereof
A technology of fluoroborate dipyrromethene and dyes, which is applied in the field of dyes to achieve the effects of high yield, sharp peak shape and high strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
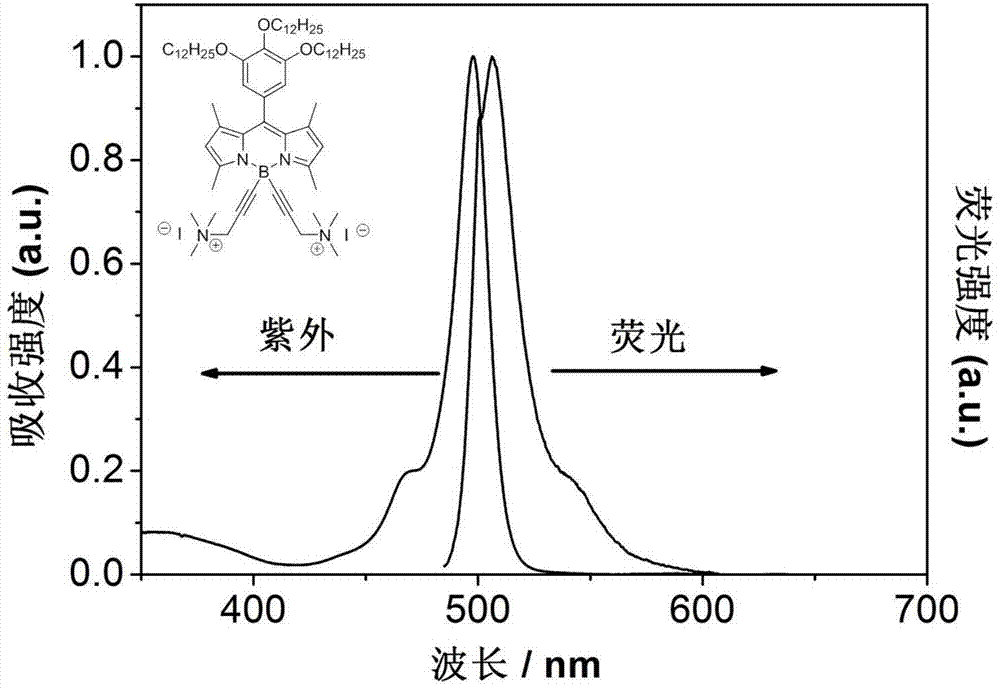
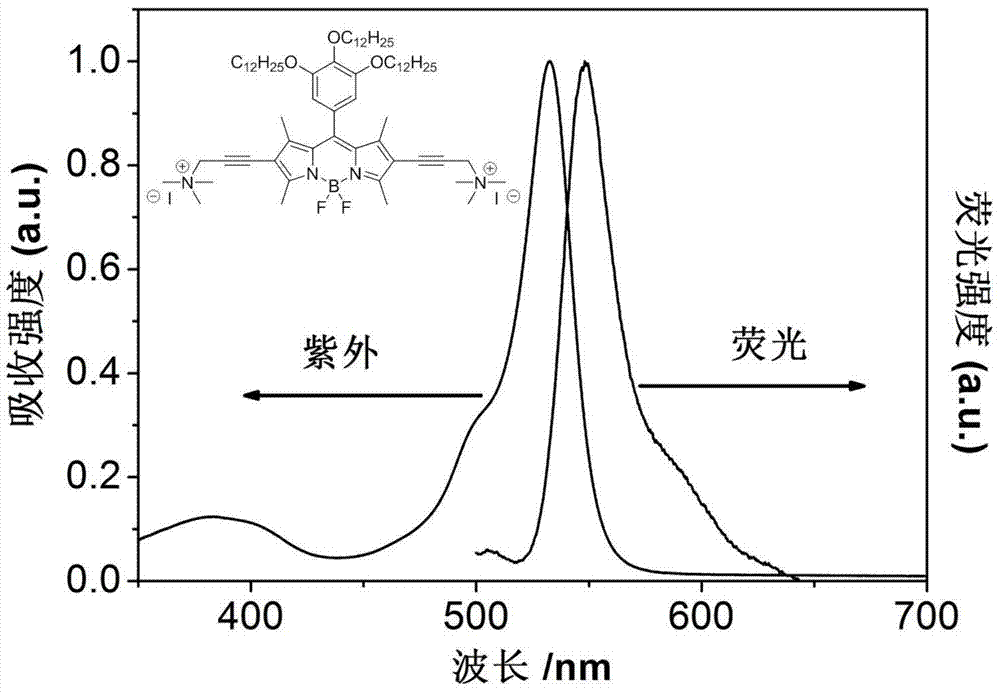
[0032] Synthesis of 4,4-difluoro-1,3,5,7-tetramethyl-8-(3,4,5-tris(dodecyloxyether))fluoroboron dipyrrole fluorescent dye: 3,4 2.0 mmol of 5-tris(dodecyloxyether) benzaldehyde and 4.0 mmol of 2,4-dimethylpyrrole were mixed in 15 ml of anhydrous dichloromethane under nitrogen atmosphere, and then 0.016 ml of trifluoroacetic acid was added. After reacting for 4 hours, add 10 ml of anhydrous tetrahydrofuran dissolved in 0.454 g of dichlorodicyanobenzoquinone, and react for 1 hour. Finally, 4.0 ml of triethylamine was added, and the reaction was continued for 15 minutes, and then 31.6 mmol of boron trifluoride ether solution was added, and the reaction was continued for 4 hours. The reaction was quenched, the layers were separated, the solvent was removed from the organic layer, and the product was obtained by column chromatography. 1 H NMR: (400MHz, CDCl 3 ):6.47(s,2H),5.99(s,2H),3.96(qd,J=19.54,6.49Hz,6H,),2.55(s,6H),1.87-1.70(m,6H),1.53(s ,6H),1.49-1.39(m,6H),1.26(s,48H),0.8...
Embodiment 2
[0035] Under nitrogen atmosphere, 0.2 g of 4,4-bis-(3-dimethylamino-1-propyne)-1,3,5,7-tetramethyl-8-(3,4,5-tri (Lauryloxyether)) fluoroborate dipyrrole fluorescent dye and 0.1ml of methyl iodide were dissolved in 20ml of anhydrous ether, and refluxed for several hours until no solid precipitated. After the reaction, spin out the solvent, add a small amount of dichloromethane to dissolve the solid, and recrystallize in ether. A dark solid was obtained by suction filtration, and 0.2 g of product was obtained after vacuum drying. 1H NMR(400MHz, CDCl3):6.50(s,2H),6.01(s,2H),4.02(d,J=6.36Hz,2H),3.91(t,J=6.53Hz,4H),3.22(s, 4H),2.78(s,6H),2.30(s,12H),1.77(dd,J=14.22,7.01Hz,6H),1.52(s,6H),1.48-1.39(m,6H),1.25(s ,48H),0.88(dd,J=6.76,3.88Hz,9H).
[0036]
Embodiment 3
[0038] Under nitrogen atmosphere, 0.14 g of 4,4-bis-(3-dimethylamino-1-propyne)-1,3,5,7-tetramethyl-8-(4-methyl)fluoroboron The dipyrrole fluorescent dye and 0.1ml of iodomethane were refluxed in anhydrous ether for several hours until no solid precipitated out. Spin out the solvent, add a small amount of dichloromethane to dissolve, and recrystallize with ether. Suction filtration yielded a dark solid, which yielded 0.14 g of product after vacuum drying for ten hours. 1H NMR (400MHz, DMSO): 2.40(s,3H), 2.65(s,6H), 3.07(s,18H), 4.30(s,4H), 6.26(s,2H), 7.18(AB,sys,2H ,J=4Hz),7.37(AB,sys,2H,J=6.4Hz).
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com