BODIPY (Boron Dipyrromethene) compound-based lysosome fluorescence probe as well as preparation method and applications thereof
A fluoroboron dipyrrole and compound technology, which is applied to the lysosome fluorescent probe based on fluoroboron dipyrrole compound and its preparation method and application field, and achieves high sensitivity, reduced photo-induced tissue damage, and high fluorescence quantum yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1.BODIPY A1:
[0029]
[0030] Benzaldehyde (0.106g, 1mmol) and 2-(2-cyano-2-acetylcarboethoxy)-1H-pyrrole (0.380g, 2mmol) were added to the reaction vessel, and dissolved in 50ml of anhydrous dichloromethane. Use a syringe to extract 0.5ml of trifluoroacetic acid (TFA) catalyst and inject it into the system, put it into the magnetic sub-stirring under the condition of anhydrous and light-proof, reflux for 4 days, and spot plate detection until the pyrrole is completely consumed. Then add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ: 0.227g, 1mmol) into the reaction solution, stir at room temperature for 30 minutes, then add excess triethylamine ( TEA) 3ml after stirring evenly, add boron trifluoride-ether solution (BF 3 ·Et 2 (0) 6ml, continue to stir and reflux for one hour to obtain a blue solution with strong red fluorescence, and spot plate detection until the uncoordinated product is exhausted, then quench the reaction with water...
Embodiment 2
[0036] The synthesis of embodiment 2.BODIPY A2:
[0037]
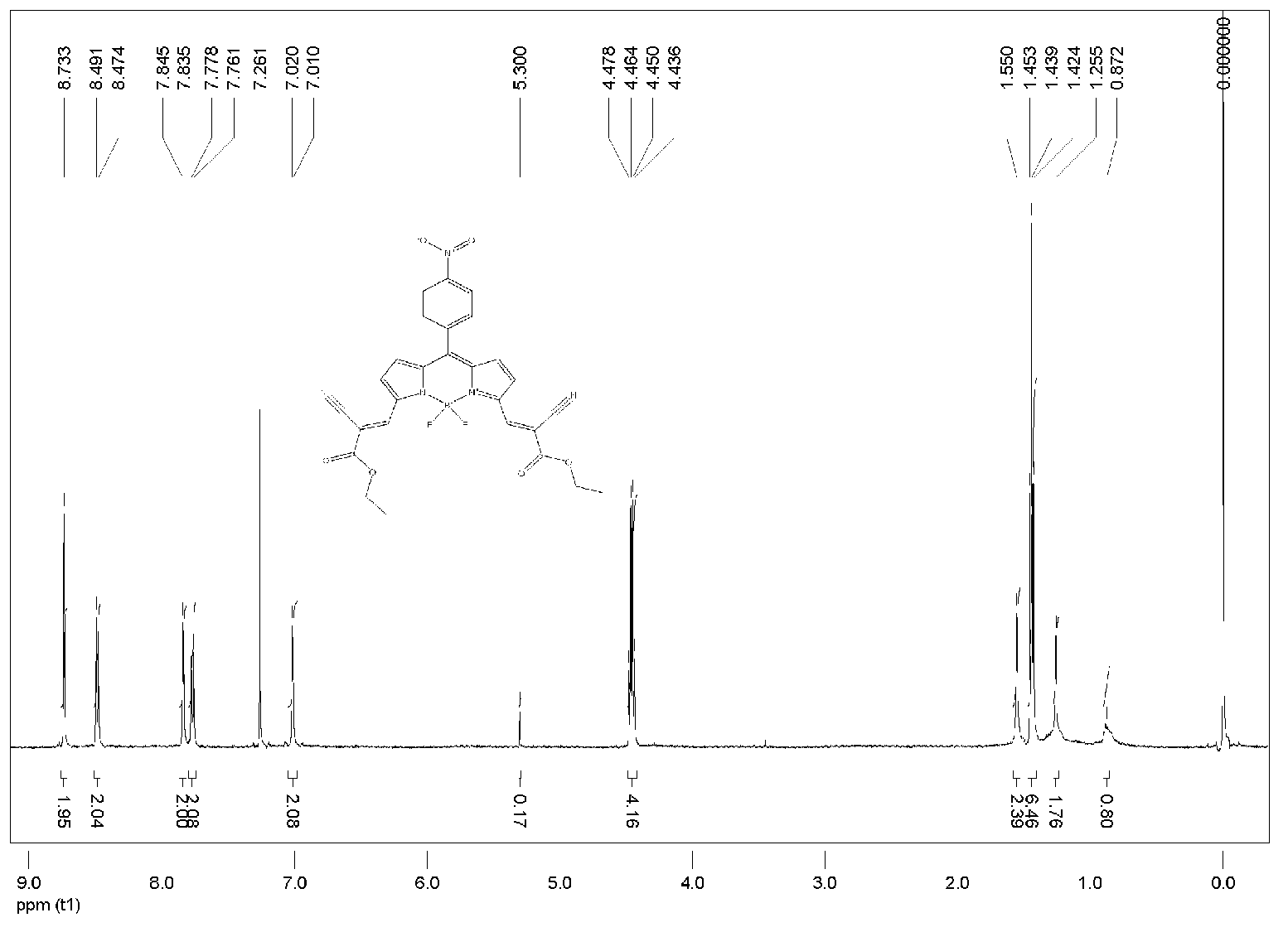
[0038] Add p-nitrobenzaldehyde (0.151g, 1mmol) and 2-(2-cyano-2-acetylcarboxylate)-1H-pyrrole (0.380g, 2mmol) to the reaction vessel, dissolve in 50ml of dry dichloromethane middle. Use a syringe to extract 0.5ml of trifluoroacetic acid (TFA) catalyst and inject it into the system, put it into the magnetic sub-stirring under the condition of anhydrous and light-proof, reflux for 7 days, and spot plate detection until the pyrrole is completely consumed. Then add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ: 0.227g, 1mmol) into the reaction solution, stir at room temperature for 30 minutes, then add excess triethylamine ( TEA) 3ml after stirring evenly, add boron trifluoride-ether solution (BF 3 ·Et 2 (0) 9ml, continue to stir and reflux for one hour to obtain a blue solution, and spot the plate to detect that the uncoordinated product is exhausted, then quench the reaction with water. The reaction mixture was w...
Embodiment 3
[0039] The synthesis of embodiment 3.BODIPY A3:
[0040]
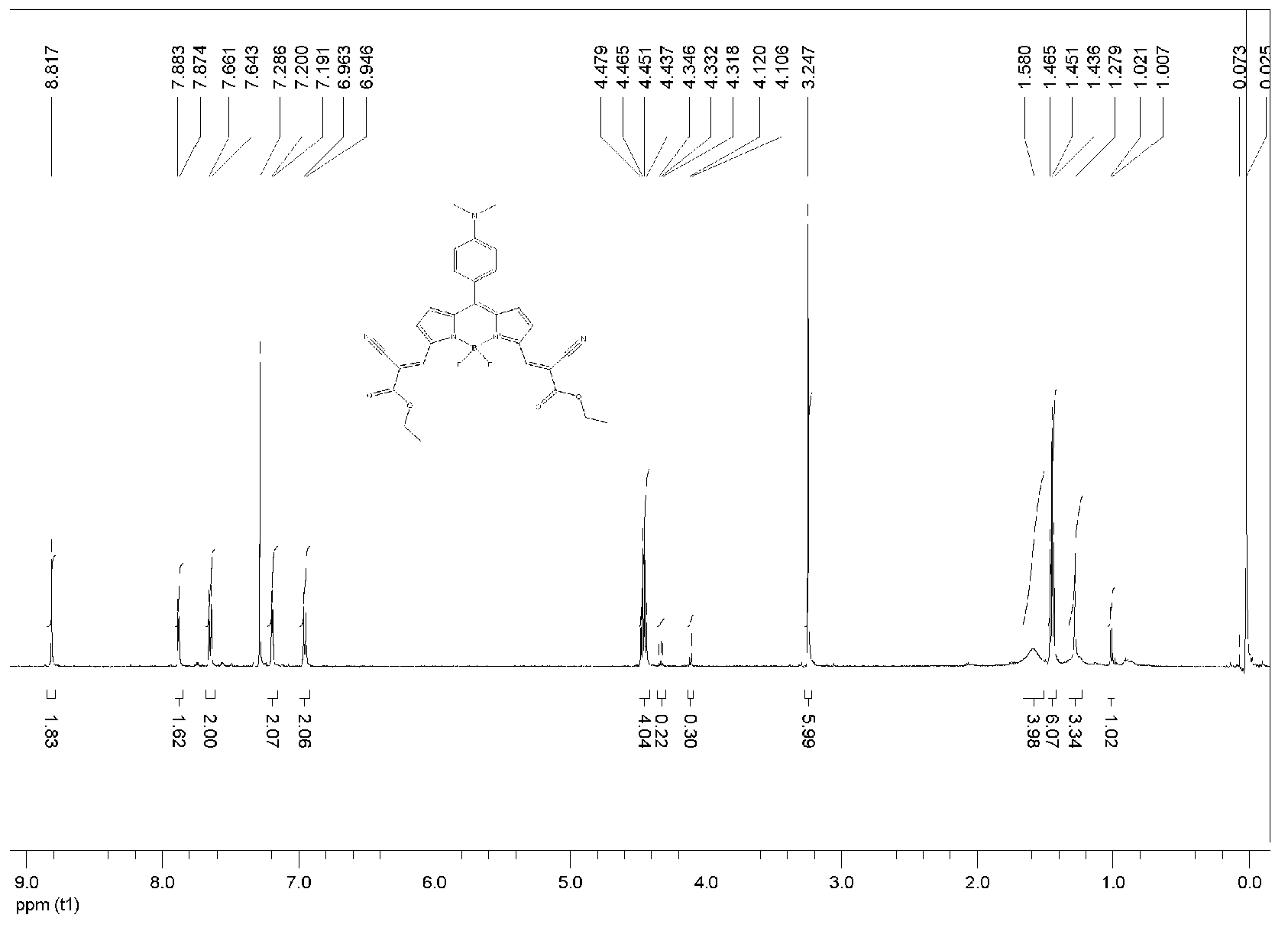
[0041] Add p-dimethylaminobenzaldehyde (0.149g, 1mmol) and 2-(2-cyano-2-acetyl ester group)-1H-pyrrole (0.380g, 2mmol) into the reaction vessel, dissolve in 50ml of anhydrous di In methyl chloride, use a syringe to extract 0.5ml of trifluoroacetic acid (TFA) catalyst and inject it into the system, put it into the magnetic sub-stirring under the condition of anhydrous and dark, and reflux for 7 days, then point the plate to detect until the pyrrole is completely consumed. Then add 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ: 0.227g, 1mmol) into the reaction solution, stir at room temperature for 30 minutes, then add excess triethylamine ( TEA) 3ml after stirring evenly, add boron trifluoride-ether solution (BF 3 ·Et 2 (0) 9ml, continue to stir and reflux for one hour to obtain a blue solution, and spot the plate to detect that the uncoordinated product is exhausted, then quench the reaction with water. The reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com