Preparation and application of fluorescent molecular probe for detecting hypochlorite ions
A fluorescent molecular probe and hypochlorite technology, applied in the field of chemical fluorescent materials, can solve problems such as interference in the interaction process between sensors and hypochlorous acid, and achieve the effects of low physiological toxicity, good effect and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
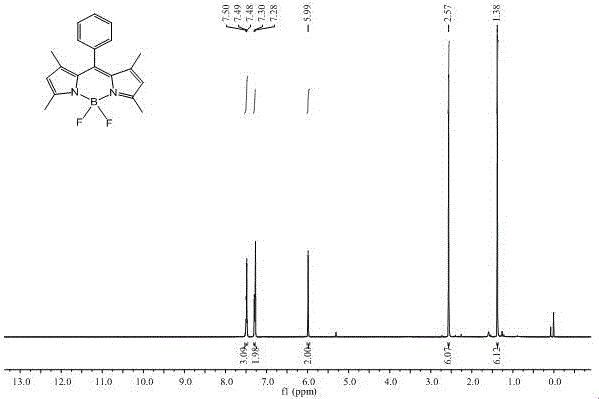
[0045] Example 1: Synthesis of intermediate compound 1 (8-phenyl-1,3,5,7-tetramethyl-fluoroborondipyrrole)
[0046]
[0047] Add 80 mL of dry dichloromethane and 0.58 mL of benzoyl chloride into a 250 mL round bottom flask, slowly add 1.1 mL of 2,4-dimethylpyrrole and 5 drops of trifluoroacetic acid (TFA ), after the addition, the solution was mixed evenly, and stirred at room temperature in the dark for 10 h. Afterwards, the sample solution was taken out, and 10 mL of triethylamine was added dropwise to the reaction in an ice bath, stirred for 15 min, and 10 mL of boron trifluoride diethyl ether (BF 3 ·Et 2 O), after the reaction, the ice bath was removed, and the reaction was continued at room temperature for 2 h. After the reaction was over, the product was washed with 100 mL saturated NaHCO 3 The solution was quenched, washed with distilled water (3×50 mL) and then extracted with dichloromethane (3×50 mL), the organic phases were combined, and anhydrous MgSO was adde...
Embodiment 2
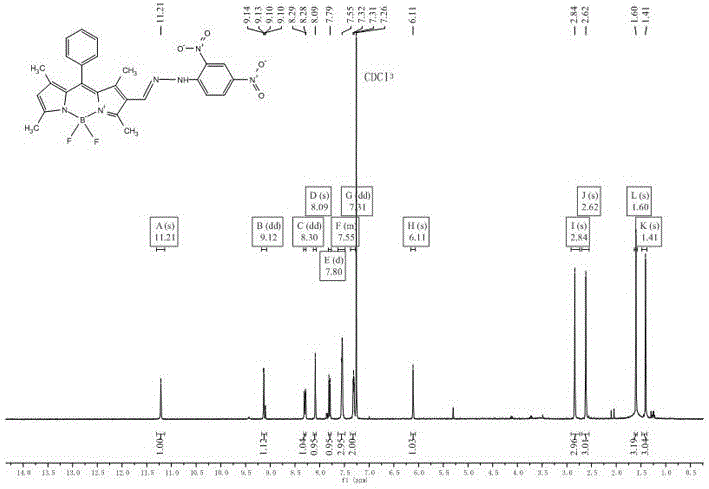
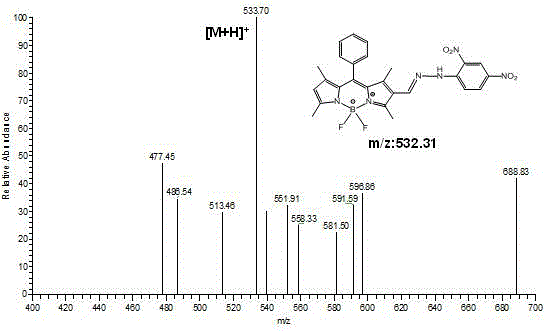
[0049] Embodiment 2: Synthesis of fluorescent probes based on BODIPY dyes
[0050] (1) Synthesis of compound 2.
[0051] Add 9.0 mL (117 mmol) of N,N-dimethylformamide (DMF) into a 250 mL round bottom flask, add 9.0 mL (117.0 mmol) of phosphorus oxychloride under the condition of ice-water bath and nitrogen protection, stir After 10 min, the ice bath was removed, returned to room temperature, and stirred for another 30 min. Then, 316 mg (1 mmol) of Compound 1 synthesized in Example 1 weighed in advance was added to 60 mL of dichloroethane (ClCH 2 CH 2 Cl), dissolved and injected into the above reaction flask, and continued to react at 45°C for 3h. Then cooled to room temperature, slowly transferred to saturated NaHCO in an ice-water bath 3 solution, add an appropriate amount of NaHCO 3 solution until the solution is weakly alkaline, and continue stirring at room temperature for 1 h. After the reaction was finished, extract with dichloromethane (3×50 mL), combine the orga...
Embodiment 3
[0054] Example 3: Synthesis of fluorescent probes based on BODIPY dyes
[0055] (2) Synthesis of compound 2.
[0056] Add 15.0 mL (195 mmol) of N,N-dimethylformamide (DMF) into a 250 mL round bottom flask, add 15.0 mL (160.0 mmol) of phosphorus oxychloride under the condition of ice-water bath and nitrogen protection, and stir for 10 min Afterwards, the ice bath was removed, returned to room temperature, and stirred for another 30 min. Then, 316 mg (1 mmol) of compound 1 synthesized in Example 1 weighed in advance was added to 80 mL of dichloroethane (ClCH 2 CH 2 Cl), dissolved and injected into the above reaction flask, and continued to react at 55°C for 5h. Then cooled to room temperature, slowly transferred to saturated NaHCO in an ice-water bath 3 solution, add an appropriate amount of NaHCO 3 solution until the solution is weakly alkaline, and continue stirring at room temperature for 1 h. After the reaction was finished, extract with dichloromethane (3×50 mL), comb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com