Synthesis and application of BODIPY dye-based hypochlorite fluorescent probe
A fluorescent probe and compound technology, applied in the synthesis and application of hypochlorous acid and fluorescent probes, can solve the problems of limited application, slow reaction process, poor selectivity, etc., and achieve shortened detection time, enhanced fluorescence intensity, and high sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
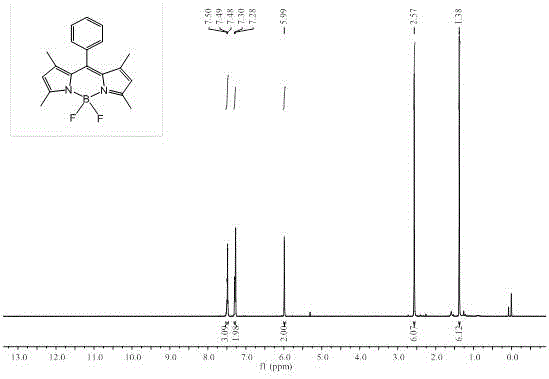
[0049] Embodiment 1: the synthesis of intermediate compound 1
[0050]
[0051] As shown in the above process, add 80 mL of dry dichloromethane and 0.58 mL of benzoyl chloride to a 250 mL round-bottomed flask, protect with nitrogen gas, slowly add 1.1 mL of 2,4-dimethylpyrrole, dropwise After adding 5 drops of trifluoroacetic acid (TFA), the mixed solution was stirred at room temperature in the dark for 10 h. Then 1.08 g of 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ) was added to the reaction, and stirring was continued for 1 h at room temperature in the dark. Afterwards, 10 mL of triethylamine was added dropwise to the reaction in an ice bath, and after stirring for 15 min, 10 mL of boron trifluoride ether (BF 3 ·Et 2 O), remove the ice bath, and continue to react at room temperature for 2 h. After the reaction is complete, wash with 100 mL of saturated NaHCO 3 The solution was quenched, washed with distilled water (3×50 mL) and then extracted with dichloromethane (3...
Embodiment 2
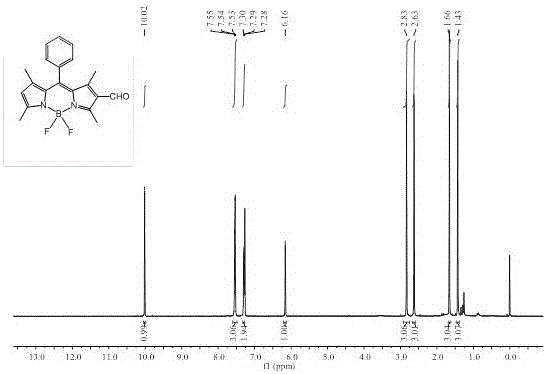
[0052] Embodiment 2: Synthesis of fluorescent probes based on BODIPY dyes
[0053] (1) Synthesis of compound 2.
[0054] Add 6 mL (78 mmol) of N,N-dimethylformamide (DMF) to a 250 mL round-bottomed flask, pass through nitrogen protection, and then add 15.0 mL (160 mmol) of phosphorus oxychloride in an ice-water bath, Stir for 10 min, remove the ice bath, return to room temperature and stir for 30 min. Then, weigh 316 mg (1 mmol) of compound 1 synthesized in Example 1 and dissolve it in 40 mL of dichloroethane (ClCH 2 CH 2 Cl), and injected into the flask, and continued to react at 40°C for 3 h. Then cooled to room temperature and slowly poured into saturated NaHCO in an ice-water bath 3 , add an appropriate amount of NaHCO 3 Continue stirring at room temperature for 1 h until the solution is slightly alkaline. After the reaction was complete, it was extracted with dichloromethane (3×50 mL), the organic phases were combined, and the solvent was spin-dried under reduced pr...
Embodiment 3
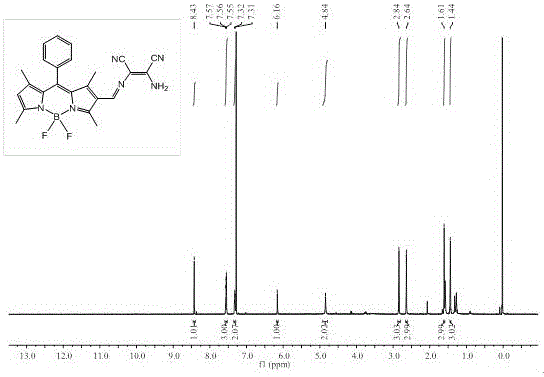
[0057] Example 3: Synthesis of fluorescent probes based on BODIPY dyes
[0058] (1) Synthesis of compound 2.
[0059] Add 15 mL (195 mmol) of N,N-dimethylformamide (DMF) to a 250 mL round-bottomed flask, pass through nitrogen protection, and then add 6.0 mL (64 mmol) of phosphorus oxychloride in an ice-water bath, Stir for 10 min, remove the ice bath, return to room temperature and stir for 30 min. Then, weigh 316 mg (1 mmol) of compound 1 synthesized in Example 1 and dissolve it in 60 mL of dichloroethane (ClCH 2 CH 2 Cl), and injected into the flask, and continued to react at 55°C for 6 h. Then cooled to room temperature and slowly poured into saturated NaHCO in an ice-water bath 3 , add an appropriate amount of NaHCO 3 Continue stirring at room temperature for 1 h until the solution is slightly alkaline. After the reaction was complete, it was extracted with dichloromethane (3×50 mL), the organic phases were combined, and the solvent was spin-dried under reduced press...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com