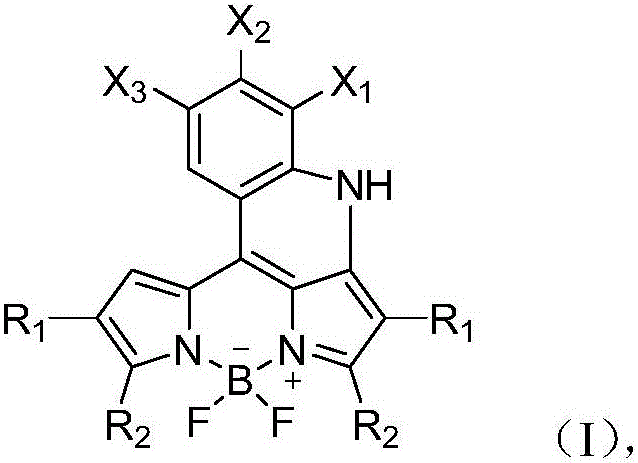
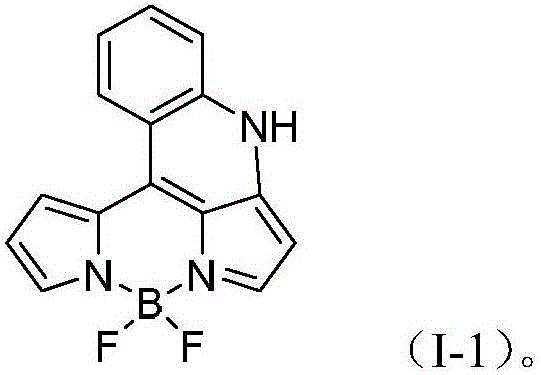
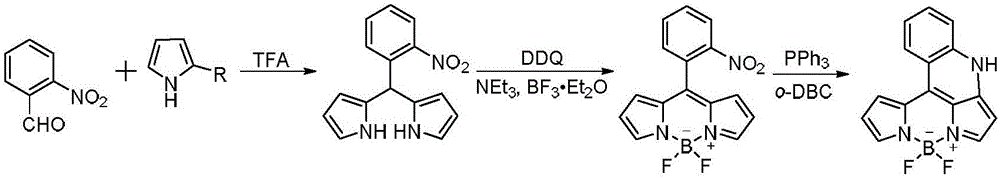BODIPY fluorescent dye, and preparation method and application thereof
A technology of fluorobodipyrrole and fluorescent dyes, which is applied in the direction of luminescent materials, azo dyes, organic dyes, etc., can solve the problem of not being able to change the absorption and emission wavelengths, achieve excellent photophysical and chemical properties, be easy for industrial production, and require equipment low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] In a (100mL) dry round bottom flask, add 2-nitrobenzaldehyde (2g, 13mmol), under the protection of nitrogen, add pyrrole (35mL, 494mmol), then slowly add trifluoroacetic acid (245μL, 1.3 mmol), keep the reaction for 1 hour after the dropwise addition is completed. Then directly carry out vacuum distillation to distill off pyrrole, and the obtained black oil is purified by silica gel column chromatography (80% dichloromethane / petroleum ether) to obtain 1.92 g of imine product.
[0031] Weigh the imine product (500mg, 1.87mmol) and dissolve it with 100mL of dry dichloromethane, add DDQ (425mg, 1.87mmol) in batches, after stirring for 1 hour, slowly add 2mL (14.42mmol) of dry triethylamine, and react After 10 minutes, 2.2 mL (17.44 mmol) of BF was added dropwise 3 ·OEt 2 , Stir the reaction at room temperature for about 1 h to complete the reaction. Add 15 mL of distilled water to quench the reaction, extract with dichloromethane, and use anhydrous Na 2 SO ...
Embodiment 2
[0038]
[0039]In a dry (100 mL) round-bottomed flask, 5-fluoro 2-nitrobenzaldehyde (2 g, 11.8 mmol) was added, under nitrogen, pyrrole (17 mL, 236 mmol) was added, and trifluoroacetic acid was slowly added dropwise (5 μL, 0.02 mmol), keep the reaction for 2 hours after the dropwise addition is completed. Then directly carry out vacuum distillation to distill off pyrrole, and the obtained black oil is purified by silica gel column chromatography (80% dichloromethane / petroleum ether) to obtain 2.2 g of dark green imine product.
[0040] Weigh the imine product (300mg, 1.1mmol) and dissolve it with 100mL of dry n-hexane, add DDQ (239mg, 1.1mmol) in batches, after stirring for 2 hours, slowly add dry triethylamine (458μL, 3.3mmol), and react After 10 minutes, add BF dropwise 3 ·OEt 2 (2.2mL, 17.44mmol), stirred at room temperature for about 2h to complete the reaction. Add 15 mL of distilled water to quench the reaction, extract with n-hexane, and use anhydrous Na for the o...
Embodiment 3
[0047]
[0048] In a (100 mL) dry round-bottom flask, add 4-chloro-2-nitrobenzaldehyde (1 g, 5.4 mmol), under nitrogen protection, add pyrrole (14.2 mL, 204.8 mmol), then slowly add three Fluoroacetic acid (143 μL, 0.54 mmol), keep the reaction for 3 hours after the dropwise addition is completed. Then directly carry out vacuum distillation to distill off the pyrrole to obtain a brownish-yellow oil which is purified by silica gel column chromatography (80% dichloromethane / petroleum ether) to obtain 1.66 g of a brownish-yellow solid imine product.
[0049] Weigh the imine product (600mg, 1.99mmol) and dissolve it with 100mL dry toluene, add DDQ (452mg, 1.99mmol) in batches, after stirring for 3 hours, slowly add dry triethylamine (2.5mL, 18.03mmol), react After 10 minutes, add BF dropwise 3 ·OEt 2 (1.30mL, 10mmol), stirred at room temperature for about 3h to complete the reaction. Add 2 mL of distilled water to quench the reaction, extract with toluene, and use anhydrous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com