Dipyrrol borane (BODIPY) and preparation method and application thereof
A compound and compound technology, applied in the field of dipyrrole borane compound and its preparation, can solve problems such as inconvenient operation, increased cost for consumers, weak absorption of detection reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Preparation of compound Ia (1,7-bis(3-methoxy-3-propionyl)-3,5-diethoxycarbonyl-2,6-dimethyl-dipyrrole borane), the relevant reaction scheme is as follows :
[0096]
[0097] 20.3 g (80 mmol) of dipyrrolyl ester (compound 1) was dissolved in 100 ml of ethyl acetate. 12.8g of liquid bromine (1eq.) was added dropwise within 15min, and after stirring for 1 hour, a pale yellow precipitate was precipitated. The above reaction mixture was spin-dried, and the solid was dissolved in 100ml of methanol, and 2ml of HCl was added to reflux for 1 hour, then cooled to 0°C and washed with 10%NH 3 ·H 2O adjusted the pH=7, and a large amount of solids were precipitated. The above solid was filtered off and recrystallized from 70:30 methanol:water solution. Pure compound (2) was obtained. 9.8g (20mmol) of compound (2) was dissolved in 60ml of dry dichloromethane, and 4.5g (1eq.) DDQ (English name of dichlorodicyanobenzoquinone: 2,3-Dichloro-5,6-dicyano -1,4-benzoquinone) was add...
Embodiment 2
[0099] Preparation of compound Ib (1,7-bis(3-methoxy-3-propionyl)-3,5-diethyl-2,6-dimethyl-dipyrrole borane), the relevant reaction scheme is as follows:
[0100]
[0101] 15.6 g (80 mmol) of dipyrrolyl ester (compound 1) was dissolved in 100 ml of ethyl acetate. 12.8g of liquid bromine (1eq.) was added dropwise within 15min, and after stirring for 1 hour, a pale yellow precipitate was precipitated. The above reaction mixture was spin-dried, and the solid was dissolved in 100ml of methanol, and 2ml of HCl was added to reflux for 1 hour, then cooled to 0°C, and washed with 10%NH 3 ·H 2 O adjusted the pH=7, and a large amount of solids were precipitated. The above solid was filtered off and recrystallized with 70:30 methanol:water solution to obtain pure compound (2). 7.5g (20mmol) of compound (2) was dissolved in 60ml of dry dichloromethane, and 4.5g (1eq.) DDQ (dichlorodicyanobenzoquinone English name:
[0102] 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone) was added to the...
Embodiment 3
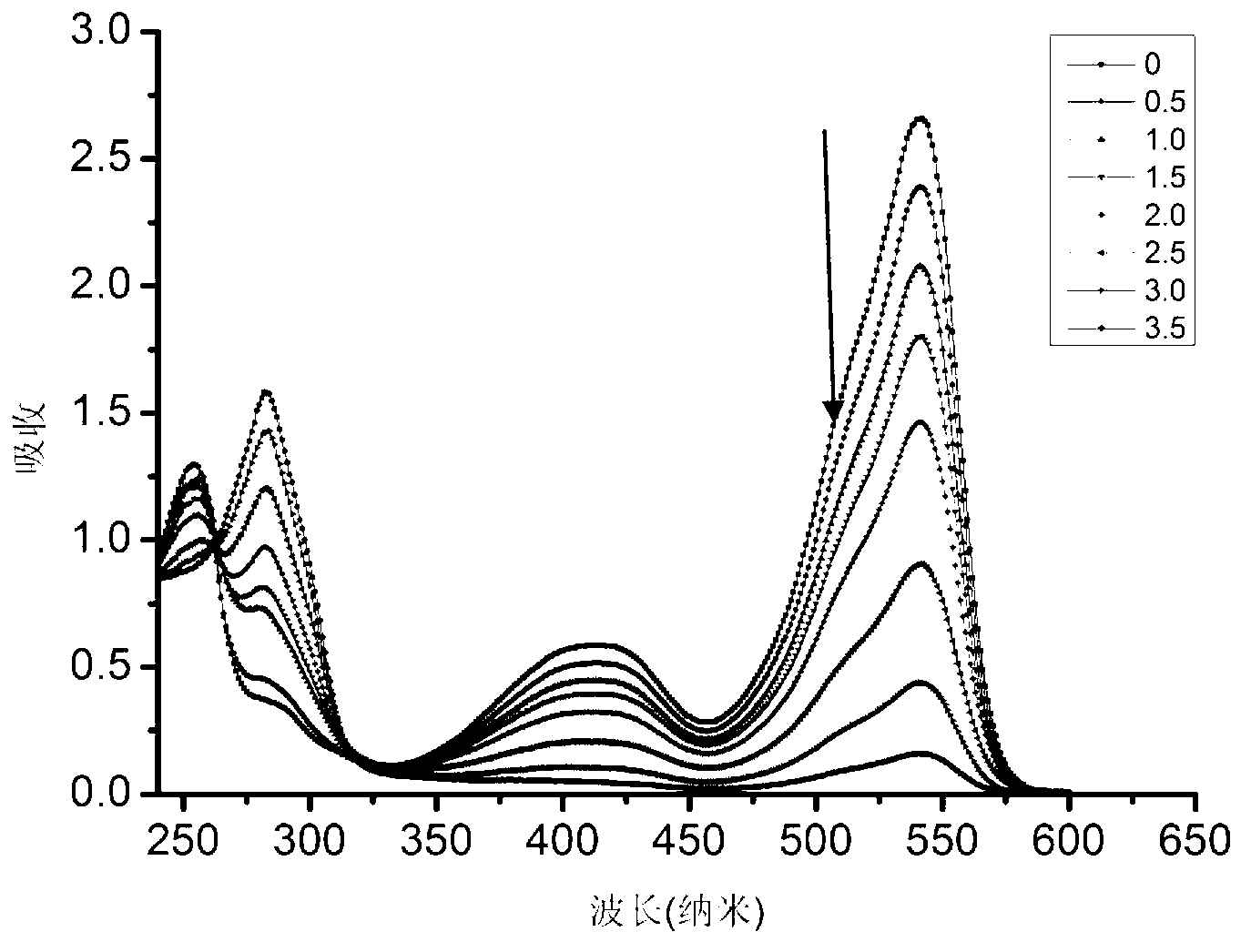
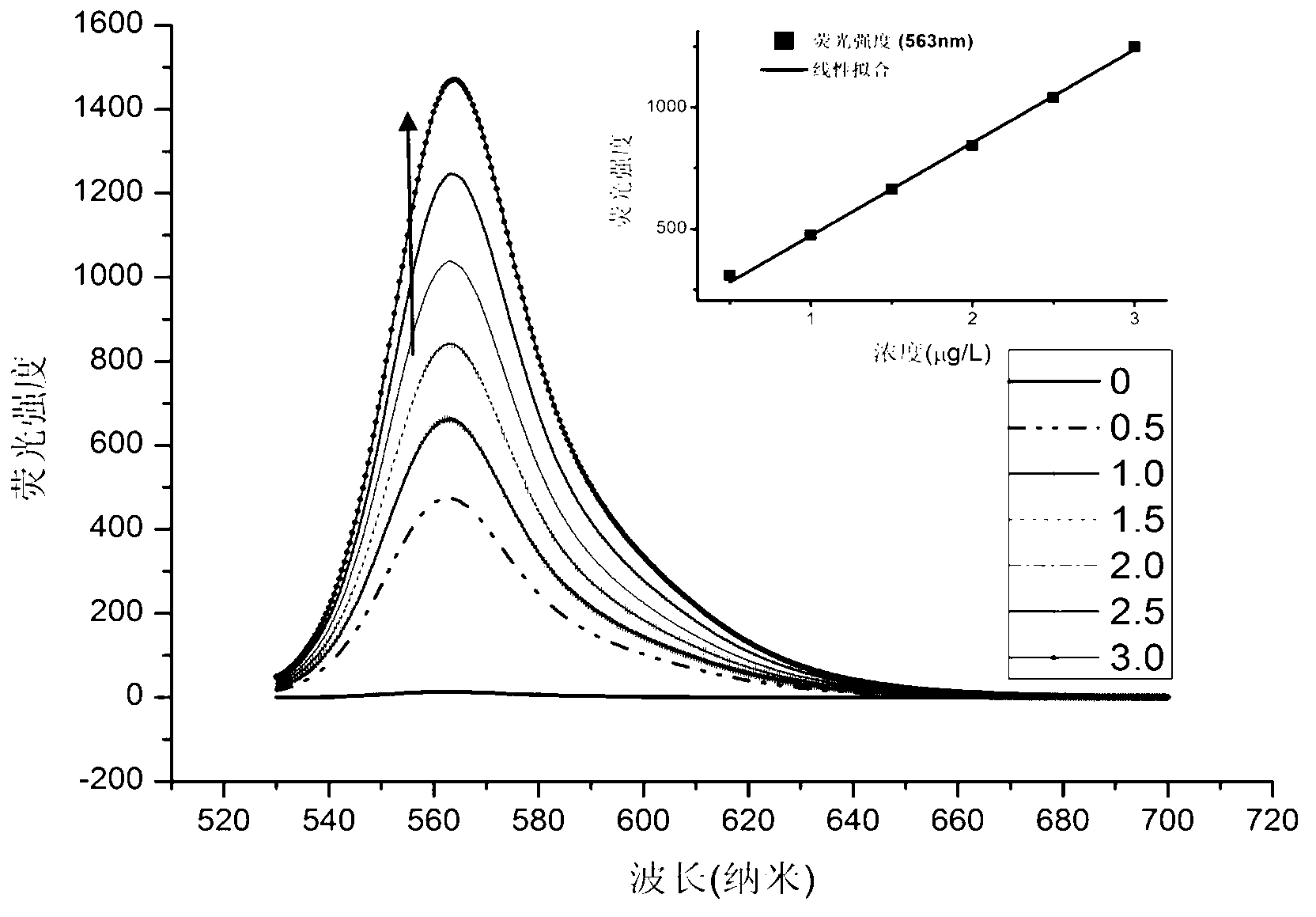
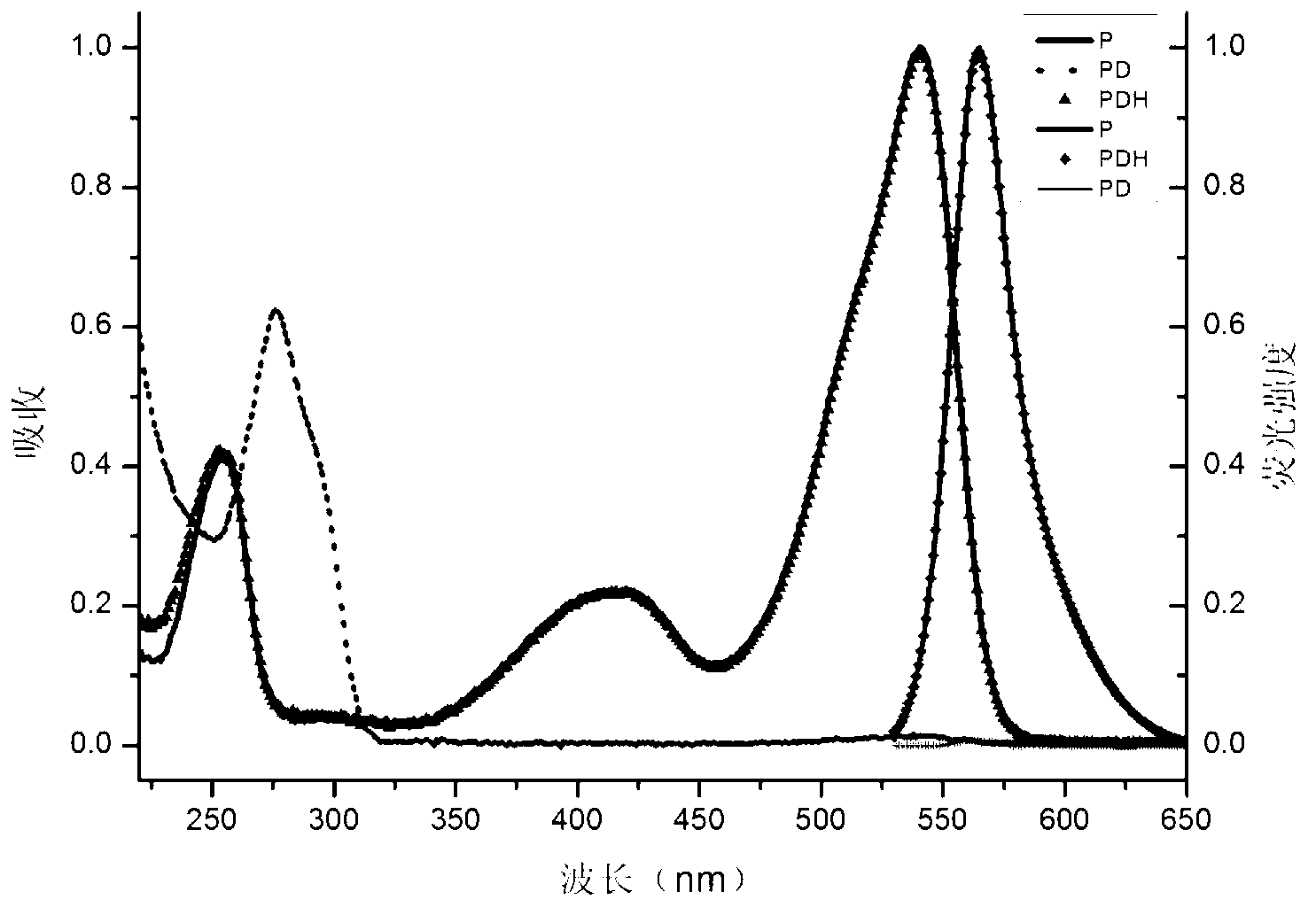
[0104] Prepare the formaldehyde detection solution containing complex IIaa from compound Ia and hydrazine hydrate:
[0105]
[0106] The compound Ia prepared in Example 1 was dissolved in an ethanol solution to form a 1mmol / L solution; after 80% hydrazine hydrate was diluted 10% with ethanol, it was made into an 8% dilute solution, and was added dropwise to In the above compound Ia solution, the solution changed from red to light red; after the above solution was placed in a dark room or refrigerator (4°C) for 24 hours, the solution became colorless, and the formaldehyde detection solution was obtained. Complex IIaa: 1 H NMR (400MHz,THF)δ5.23(s,1H),4.32–4.08(m,4H),3.57(s,6H),2.98–2.82(m,2H),2.82–2.69(m,2H), 2.63–2.50(m,2H),2.50–2.35(m,2H),2.24(s,6H),1.32(t,J=7.1Hz,6H).
[0107] The high-resolution mass spectrometry data of the colorless solution after adding hydrazine are shown in the following table:
[0108]
[0109]
[0110] The crystal structure of complex IIaa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com