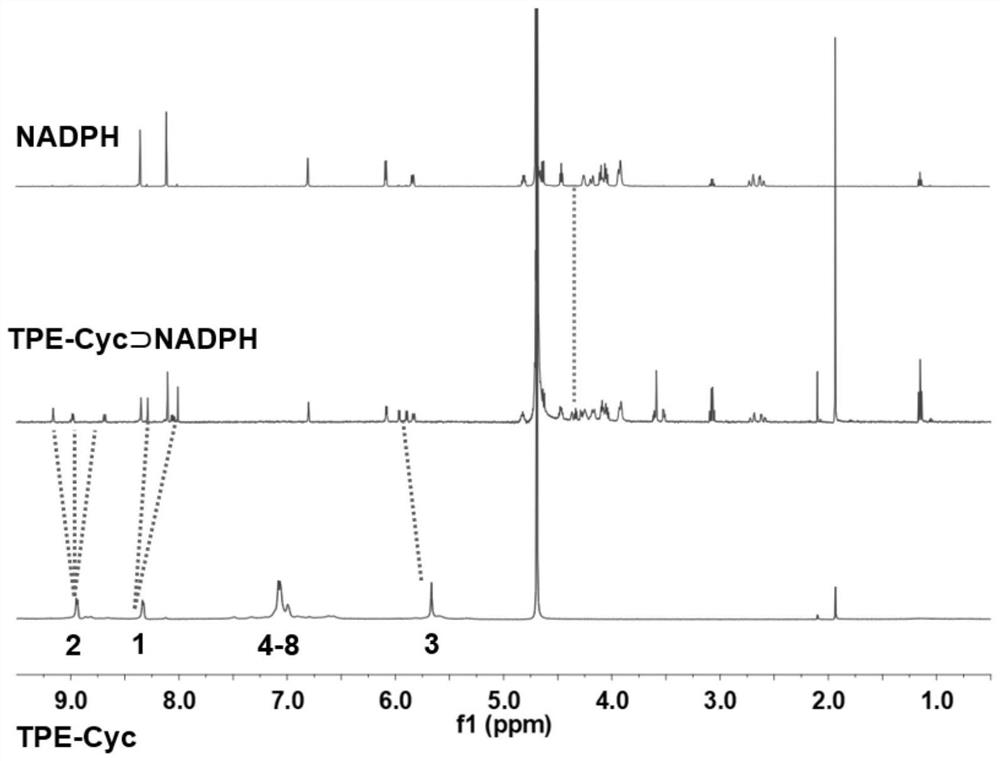
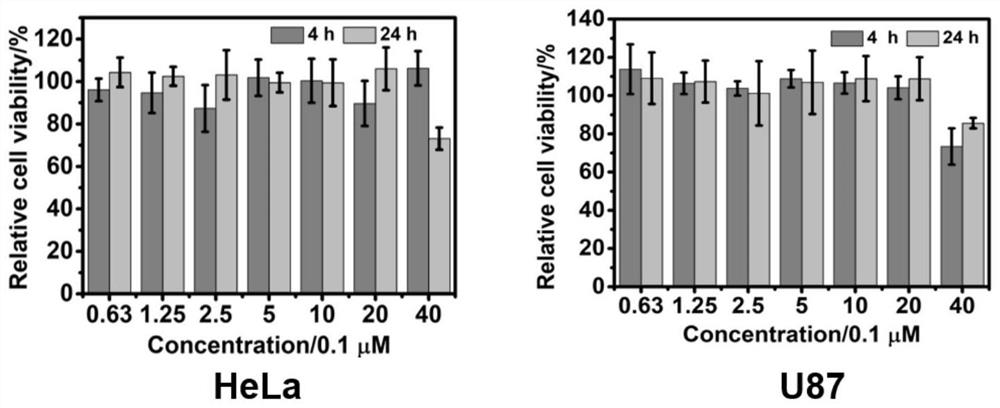
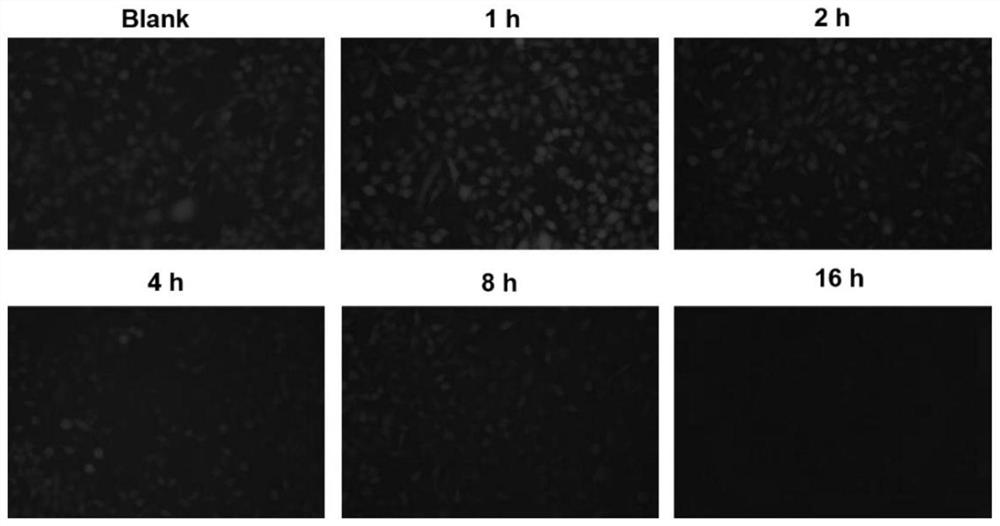
Application of hydrophilic tetra-cation cyclophane constructed based on TPE
A cationic and hydrophilic technology, applied in the direction of medical preparations containing active ingredients, organic active ingredients, instruments, etc., to achieve the effect of improving antioxidant capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the synthesis of compound 3
[0042]at 0°C, N 2 Under atmosphere, 37.1 mL of n-butyllithium (1.6M) was added to a solution of compound 1 (10 g, 59.4 mmol) in THF (100 mL), and the resulting orange-red solution was stirred at 0° C. for 30 min. Then, compound 2 (6.3g, 29.7mmol) was dissolved in THF (20mL) to obtain a THF solution of compound 2, and then the solution of compound 2 was added dropwise to the mixed solution of compound 1 and n-butyllithium, and the mixed solution was heated To room temperature, continue to stir for 6h. The reaction was quenched with 60 mL of saturated aqueous ammonium chloride. Extract the organic phase with DCM and anhydrous Na 2 SO 4 dry. The resulting crude product was dissolved in 20 mL of toluene, a catalytic amount of p-toluenesulfonic acid (342 mg, 1.8 mmol) was added, and the reaction was refluxed at 90 ° C for 5 h. Molecular sieve dehydration unit separates the generated water. With mass fraction 10% NaHCO 3 Wa...
Embodiment 2
[0043] Embodiment 2: the synthesis of compound 4
[0044] in N 2 Under atmosphere, compound 3 (1.1g, 2.83mmol) was dissolved in CCl 4 (20mL), then NBS (1.5g, 8.49mmol) and dibenzoyl peroxide (50mg, 0.2mmol) were added, and finally the mixture was heated to reflux at 70°C for 12h. After the reaction, the mixture was filtered to remove solid impurities. Extraction with DCM, organic phase with anhydrous Na 2 SO 4 dry. The solvent was removed by rotary evaporation, and the resulting crude product was purified by column chromatography (silica gel; the volume ratio of petroleum ether to ethyl acetate was 10:1 as the eluent), and the eluate containing the target compound was collected, spin-dried, and then 50°C After vacuum drying for 12 h, compound 4 (0.9 g, yield 60%) was obtained.
Embodiment 3
[0045] Embodiment 3: the synthesis of compound 5,
[0046] 4,4'-bipyridine (2.0 g, 12.8 mmol) was dissolved in 20 mL of acetonitrile, and heated to reflux at 75°C. A solution of compound 4 (1.2 mg, 2.3 mmol) in acetonitrile (5 mL) was then added dropwise to the bipyridine solution. Reflux at 75°C for 3 days. The reacted mixture was filtered, and the filter cake was washed 3 times with acetonitrile. After vacuum drying at 50° C. for 12 h, compound 5 (1.7 g, yield 90%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com