Dipyrrole borane compound (bodipy) and its preparation method and application
A compound and complex technology, applied in the field of dipyrrolidone compounds and their preparation, can solve the problems of long time, inconvenient operation, expensive instruments and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Preparation of compound Ia (1,7-bis(3-methoxy-3-propionyl)-3,5-diethoxycarbonyl-2,6-dimethyl-dipyrrole borane), the relevant reaction scheme is as follows :
[0096]
[0097] 20.3 g (80 mmol) of dipyrrolyl ester (compound 1) was dissolved in 100 ml of ethyl acetate. 12.8g of liquid bromine (1eq.) was added dropwise within 15min, and after stirring for 1 hour, a pale yellow precipitate was precipitated. The above reaction mixture was spin-dried, and the solid was dissolved in 100ml of methanol, and 2ml of HCl was added to reflux for 1 hour, then cooled to 0°C, and washed with 10%NH 3 ·H 2O adjusted the pH=7, and a large amount of solids were precipitated. The above solid was filtered off and recrystallized from 70:30 methanol:water solution. Pure compound (2) was obtained. 9.8g (20mmol) of compound (2) was dissolved in 60ml of dry dichloromethane, and 4.5g (1eq.) DDQ (English name of dichlorodicyanobenzoquinone: 2,3-Dichloro-5,6-dicyano -1,4-benzoquinone) was ad...
Embodiment 2
[0099] Preparation of compound Ib (1,7-bis(3-methoxy-3-propionyl)-3,5-diethyl-2,6-dimethyl-dipyrrole borane), the relevant reaction scheme is as follows:
[0100]
[0101] 15.6 g (80 mmol) of dipyrrolyl ester (compound 1) was dissolved in 100 ml of ethyl acetate. 12.8g of liquid bromine (1eq.) was added dropwise within 15min, and after stirring for 1 hour, a pale yellow precipitate was precipitated. The above reaction mixture was spin-dried, and the solid was dissolved in 100ml of methanol, and 2ml of HCl was added to reflux for 1 hour, then cooled to 0°C, and washed with 10%NH 3 ·H 2 O adjusted the pH=7, and a large amount of solids were precipitated. The above solid was filtered off and recrystallized with 70:30 methanol:water solution to obtain pure compound (2). 7.5g (20mmol) of compound (2) was dissolved in 60ml of dry dichloromethane, and 4.5g (1eq.) DDQ (dichlorodicyanobenzoquinone English name:
[0102] 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone) was added to the...
Embodiment 3
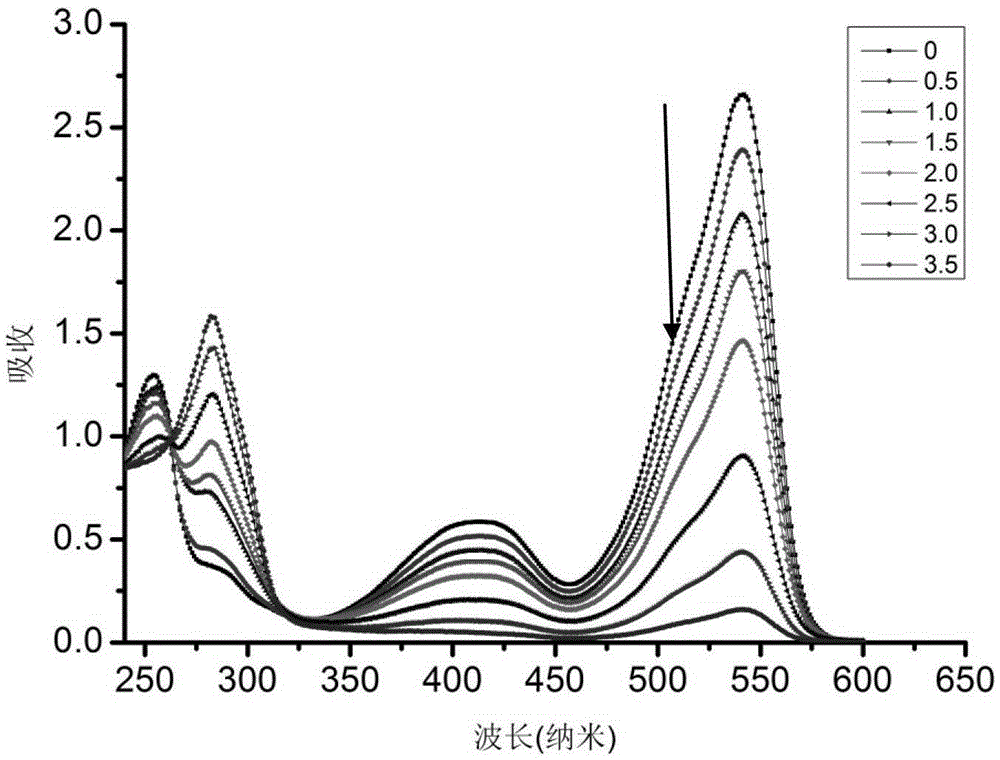
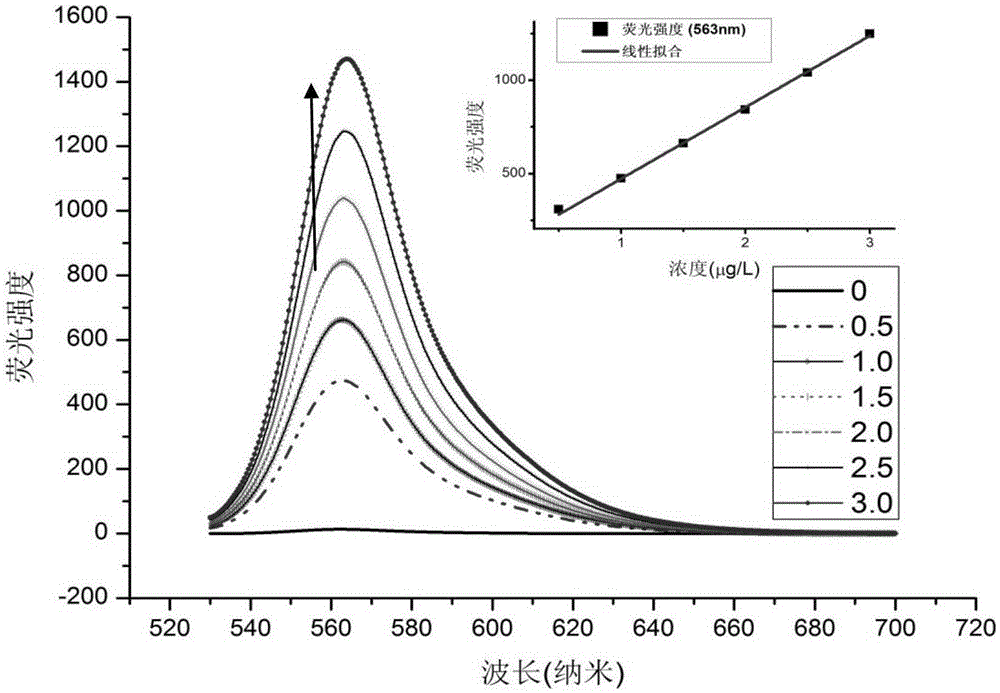
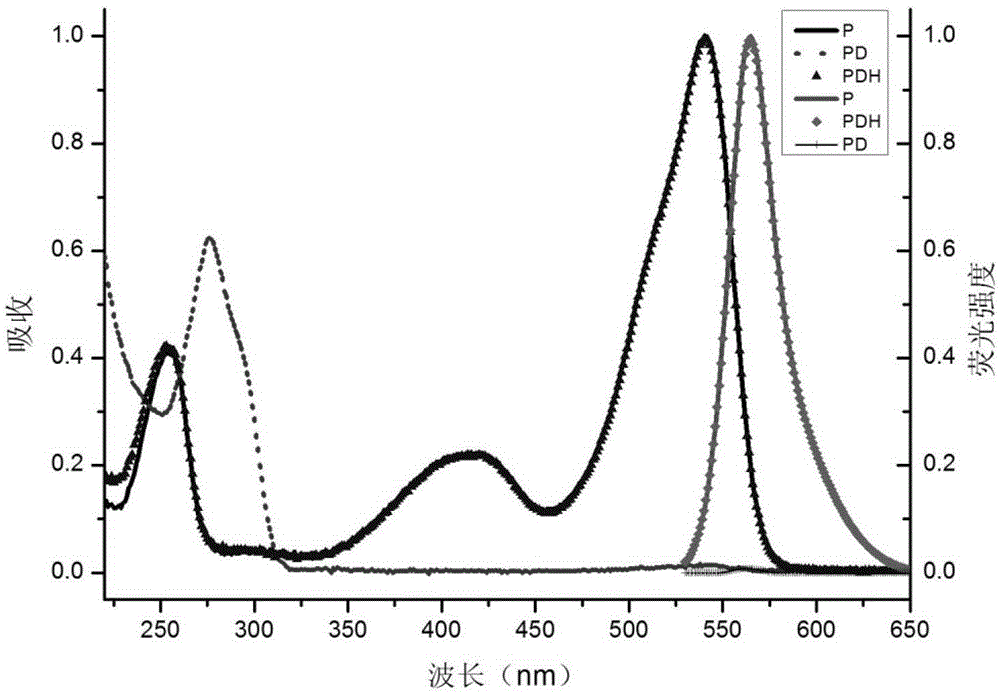
[0104] Prepare the formaldehyde detection solution containing complex IIaa from compound Ia and hydrazine hydrate:
[0105]
[0106] The compound Ia prepared in Example 1 was dissolved in an ethanol solution to form a 1mmol / L solution; after 80% hydrazine hydrate was diluted 10% with ethanol, it was made into an 8% dilute solution, and was added dropwise to In the above compound Ia solution, the solution changed from red to light red; after the above solution was placed in a dark room or refrigerator (4°C) for 24 hours, the solution became colorless, and the formaldehyde detection solution was obtained. Complex IIaa: 1 HNMR (400MHz,THF)δ5.23(s,1H),4.32–4.08(m,4H),3.57(s,6H),2.98–2.82(m,2H),2.82–2.69(m,2H),2.63 –2.50(m,2H),2.50–2.35(m,2H),2.24(s,6H),1.32(t,J=7.1Hz,6H).
[0107] The high-resolution mass spectrometry data of the colorless solution after adding hydrazine are shown in the following table:
[0108]
[0109]
[0110] The crystal structure of complex IIaa ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com