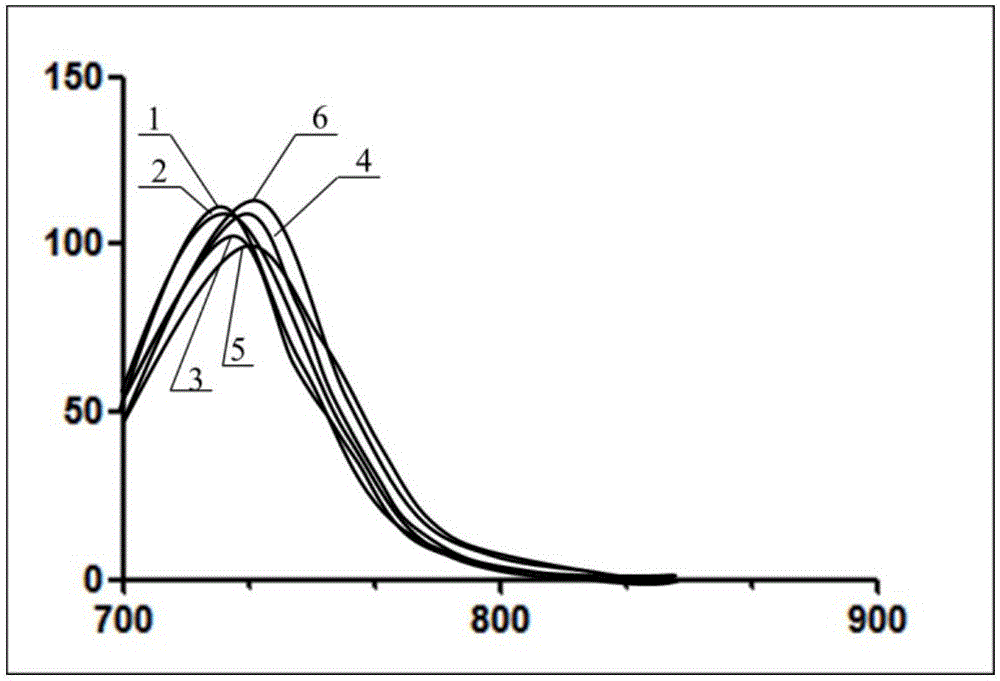
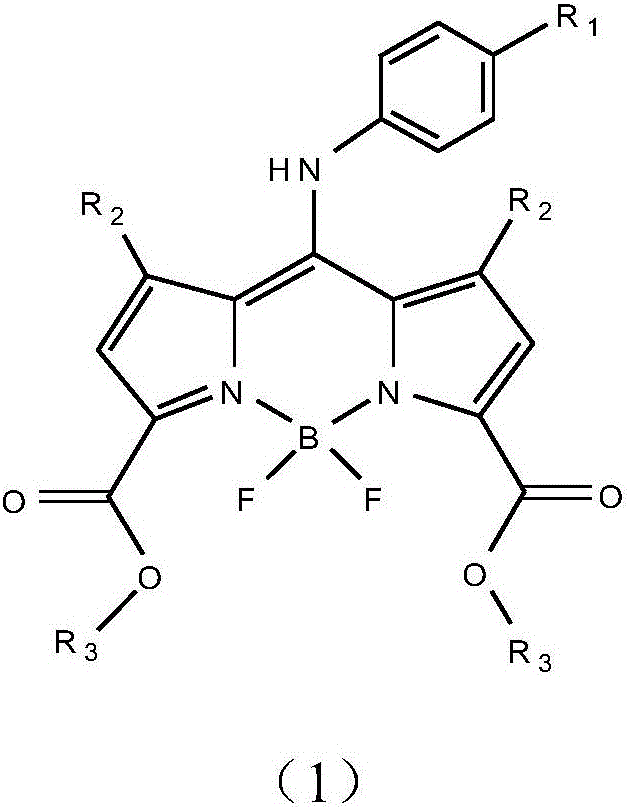
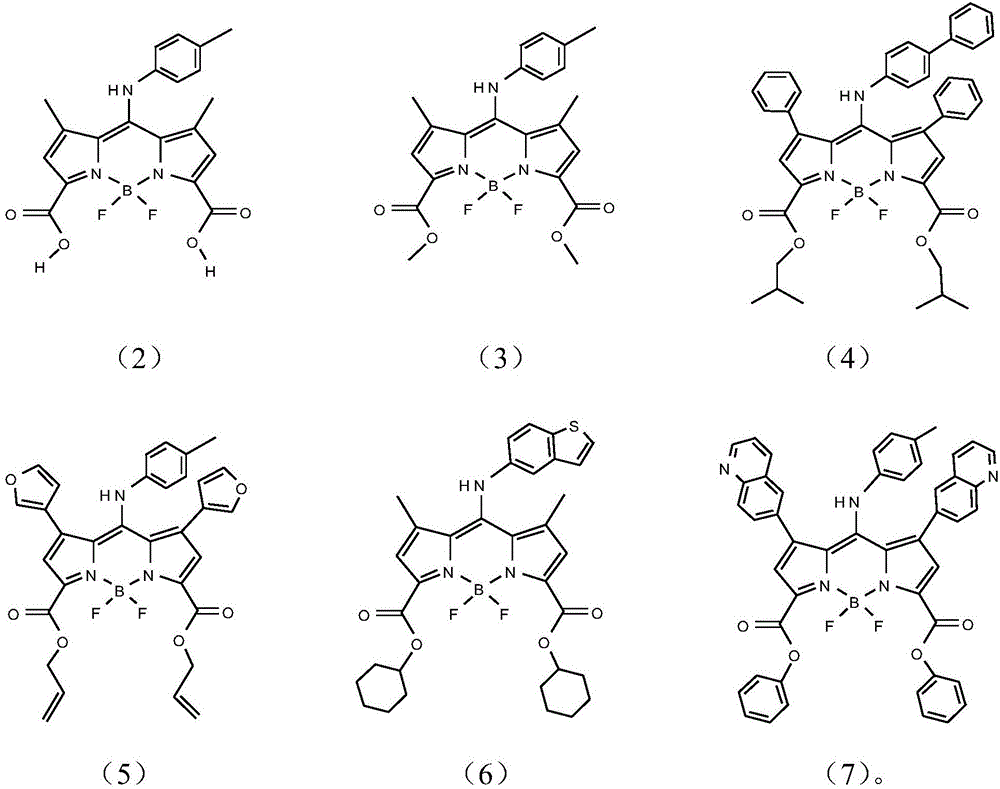
Infrared BODIPY fluorochrome as well as preparation method and application thereof
A fluorescent dye, infrared technology, used in luminescent materials, azo dyes, organic dyes, etc., can solve the problems of low molar absorption coefficient, low fluorescence quantum yield, weak fluorescence signal, etc., to achieve high molar absorption coefficient, enhanced organic Molecular fluorescence effect, the effect of narrow fluorescence emission spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The present embodiment provides the preparation method of BODIPY fluorescent dye (2):
[0038]
[0039] The preparation route is:
[0040]
[0041] The preparation method is:
[0042] S1. Take 0.25mL of chloroacetyl chloride and 0.70mL of 4-bromo-2-pyrrole carboxylic acid into a single-necked flask containing 1.5mL of dichloromethane solvent, stir at room temperature for 10min, and detect the raw material 4-bromo - The reaction of 2-pyrrole carboxylic acid is completed to obtain intermediate A1.
[0043] S2. Measure 8mL of boron trifluoride ether solution and 5mL of triethylamine and slowly add it dropwise to the reaction solution in step S1, and keep stirring. The reaction temperature is controlled at 25-30°C. Evaporate and remove the dichloromethane solvent to obtain a viscous liquid; use silica gel column separation and purification, the developer is petroleum ether (b.p.60-90°C): ethyl acetate (v:v) ratio of 5:1, to obtain intermediate B1.
[0044] S3. Get ...
Embodiment 2
[0052] The present embodiment provides the preparation method of BODIPY fluorescent dye (3):
[0053]
[0054] The preparation route is:
[0055]
[0056] The preparation method is:
[0057] Steps S1-S5 are the same as Steps S1-S5 of Example 1 to obtain intermediates A2, B2, C2 and D2 respectively.
[0058] S6. Under the protection of nitrogen, add 0.40mmol oxalyl chloride and THF solution for dissolving 0.48mmol intermediate D2 successively in a three-necked flask equipped with a reflux condenser, temperature sensor and stirrer, and stir; 150mL of methanol is added dropwise, and the reaction solution is React overnight at 70°C; take 100mL of triethylamine in the reaction solution, filter and evaporate to obtain the crude product (3); the crude product (3) is washed twice with saturated saline and passed through a silica gel column, and the developer is petroleum ether ( b.p.60-90°C): Ethyl acetate (v:v) ratio of 5:1; finally recrystallized with absolute ethanol to obt...
Embodiment 3
[0064] The present embodiment provides the preparation method of BODIPY fluorescent dye (4):
[0065]
[0066] The preparation route is:
[0067]
[0068] The preparation method is:
[0069] Steps S1-S2 are the same as Steps S1-S2 of Example 1 to obtain intermediates A3 and B3 respectively.
[0070] S3. Take 40mL of anhydrous tetrahydrofuran and add it to a 100mL single-necked flask, weigh 0.52mmol of intermediate B3, 0.35mmol of 4-aminobiphenyl, 0.12mmol of potassium iodide and 0.72mmol of anhydrous potassium carbonate and add it to the single-necked flask ;Under the protection of nitrogen, stir the reaction at room temperature for 5h; filter, and distill under reduced pressure to remove tetrahydrofuran; use silica gel column separation and purification, the developer is petroleum ether (b.p.60-90°C): ethyl acetate (v:v) is 5: 1, and added 3mL of triethylamine to obtain intermediate C3.
[0071] S4. On the 100mL three-necked flask, equipped with a stirrer, constant p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com