Long wavelength boron dipyrromethene dye and preparation thereof
A technology of fluoroboridipyrrole and long wavelength, which is applied in the field of preparation of long-wavelength fluoroboridipyrrole dyes and their derivatives, and can solve problems such as large application potential, insufficient properties of target compounds, and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
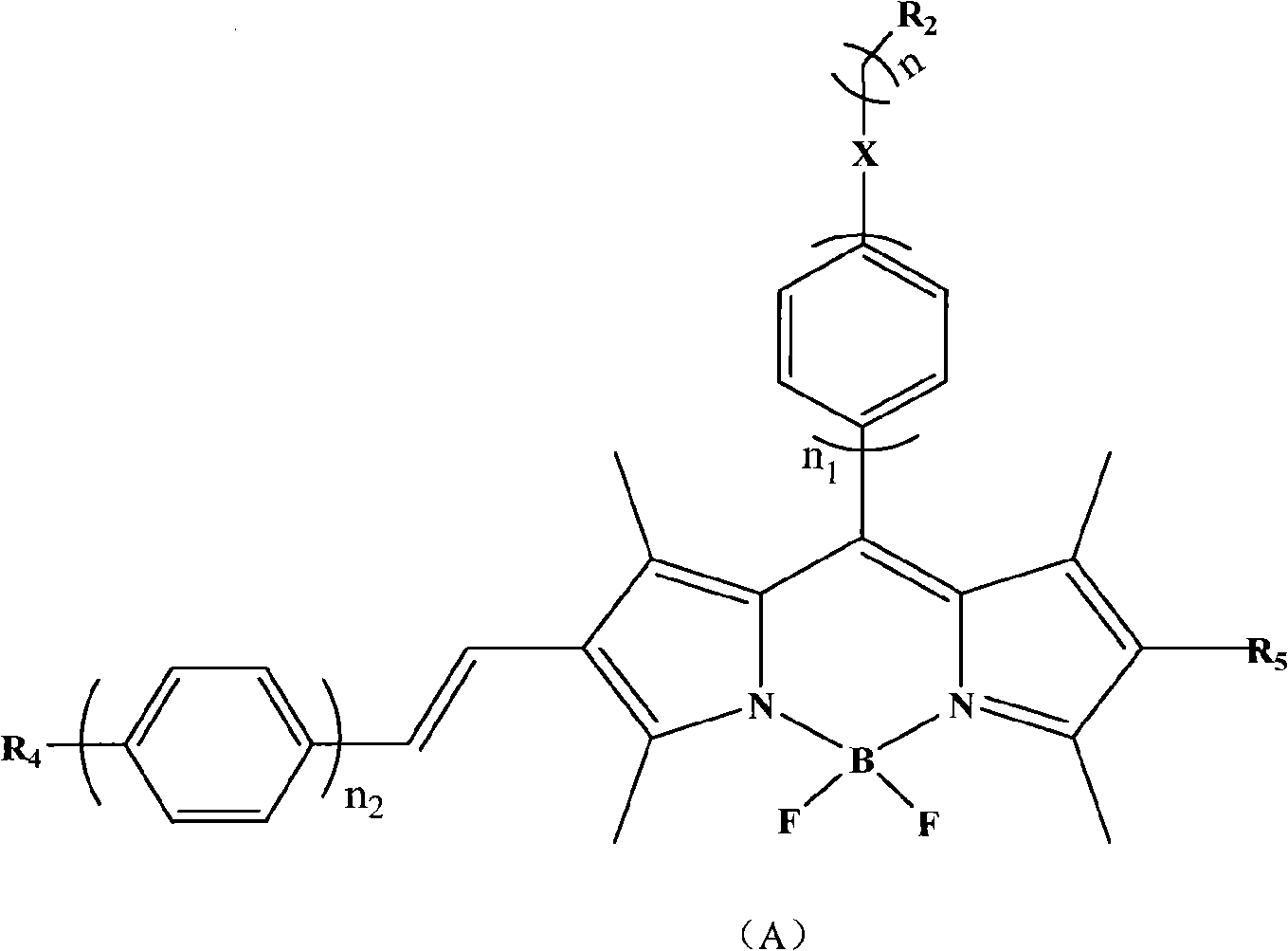
[0041] Add 0.51 mmol of dibromo-substituted fluoroborate dipyrrole, 3 times the molar amount of styrene boronic acid, 40 mL of N, N-dimethylformamide to a 100 mL two-necked bottle, keep the system at 50 ° C under stirring, react for 8 h, and pass through a silica gel column to obtain Blue-purple solid, yield 80%, HRMS[M] + : 466.2393(m / z); 1 H-NMR (400Mz, CDCl3): δ=1.37(s, 6H), 1.70(s, 3H), 2.56(s, 6H), 6.65(d, 2H), 6.84(d, 2H) 7.21-7.30(m ,10H); 13 C-NMR (100MHz, CDCl 3 ): δ=14.0, 16.4, 20.8, 106.0, 112.0, 121.8, 122.6, 125.7, 126.5, 127.6, 128.7, 129.5, 130.6, 148.7.
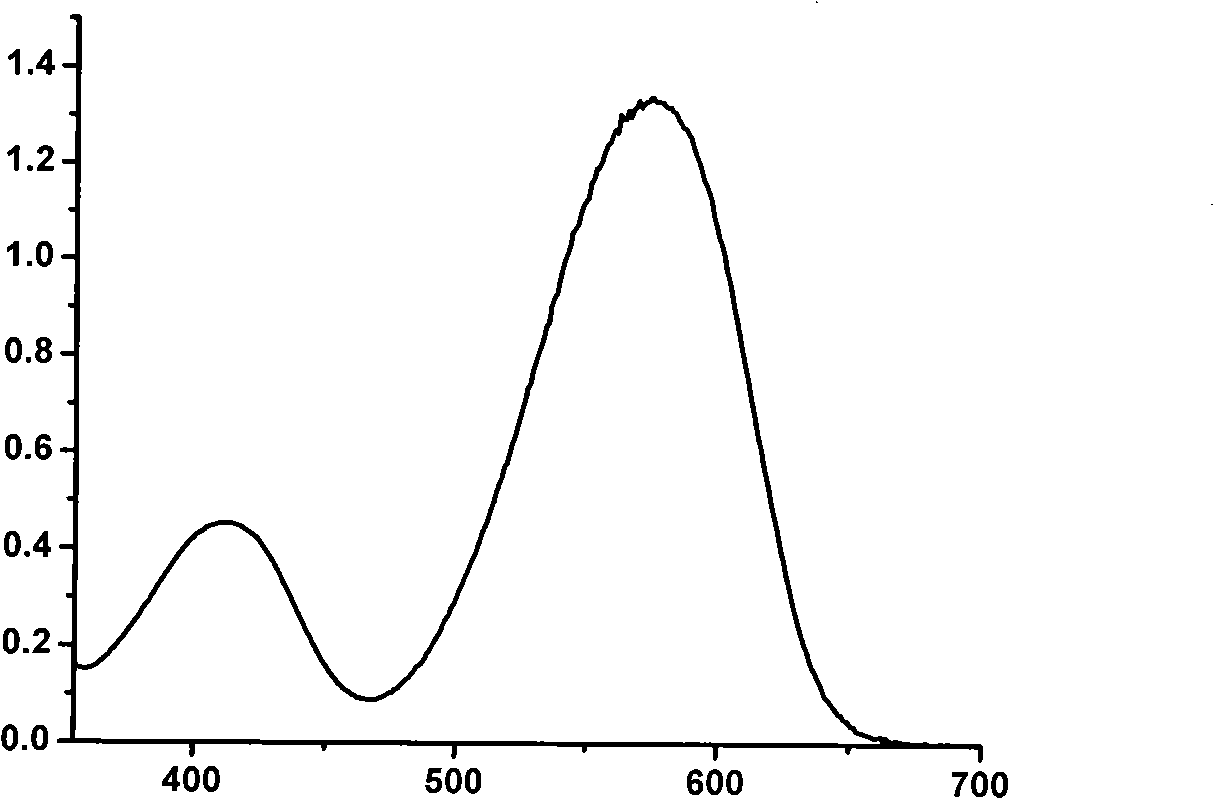
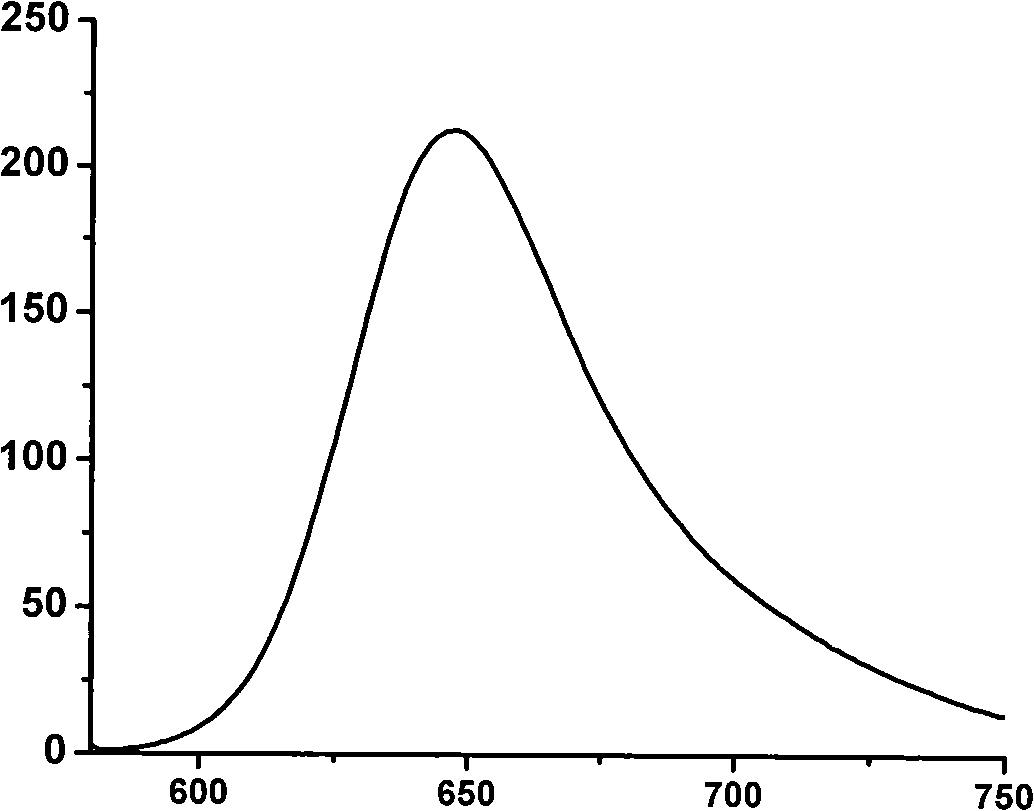
[0042] figure 1 , 2 Spectral data for this product are given. figure 1 It is the absorption spectrogram of product, and ordinate represents relative intensity, and abscissa represents wavelength, and the maximum absorption wavelength of this product is 574 nanometers. figure 2 is the fluorescence emission spectrum diagram of the product, the ordinate represents the relative intensity, and t...
Embodiment 2
[0044]
[0045] Add 0.51mmol monobromofluoroborate dipyrrole, 2.5 times the molar amount of p-methoxystyrene boronic acid, 40mL dimethyl sulfoxide to a 100mL two-necked bottle, keep the system at 60°C under stirring, react for 15h, pass through a silica gel column, A purple solid was obtained, the yield was 75%, HRMS[M] + : 456.2185(m / z); 1 H-NMR (400Mz, CDCl3): δ=1.37(s, 3H), 1.57(s, 3H), 2.26(s, 3H), 2.56(s, 3H), 3.06(s, 3H), 6.0(s, 1H), 6.63(d, 1H), 6.83(d, 1H), 7.14-7.30(m, 9H); 13 C-NMR (100MHz, CDCl 3 ):
[0046] δ=12.0, 14.4, 20.8, 25.7, 60.4, 109.0, 112.8, 116.6, 118.7, 120.1, 121.5, 122.2, 123.0, 125.4, 126.8, 127.7, 128.6, 129.4, 130.8, 131.5, 138, 139.0, 1395 140.7, 142.6.
Embodiment 3
[0048]
[0049] Add 0.51 mmol of bis-bromofluoroborate dipyrrole, 3.5 times the molar amount of hexene boronic acid, and 40 mL of 1,4-dioxane to a 100 mL two-necked bottle, keep the system at 70 ° C under stirring, react for 18 h, and pass through a silica gel column to obtain Blue-purple solid, yield 72%, HRMS[M] + : 560.3386(m / z); 1 H-NMR (400Mz, CDCl3): δ=0.8-1.2 (m, 22H), 1.57 (s, 6H), 2.56 (s, 6H), 3.06 (s, 3H), 6.0 (s, 1H), 6.63 ( d, 1H), 6.83 (d, 1H), 7.14-7.30 (m, 4H), 10.42 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com