Near-infrared two-window fluorescence probe based on Aza-BODIPY, as well as preparation and application thereof
A fluorescent probe and near-infrared technology, applied in the field of organic fluorescent probes, can solve the problems of low resolution, shallow penetration depth, poor signal-to-noise ratio, etc., and achieve good photostability and no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
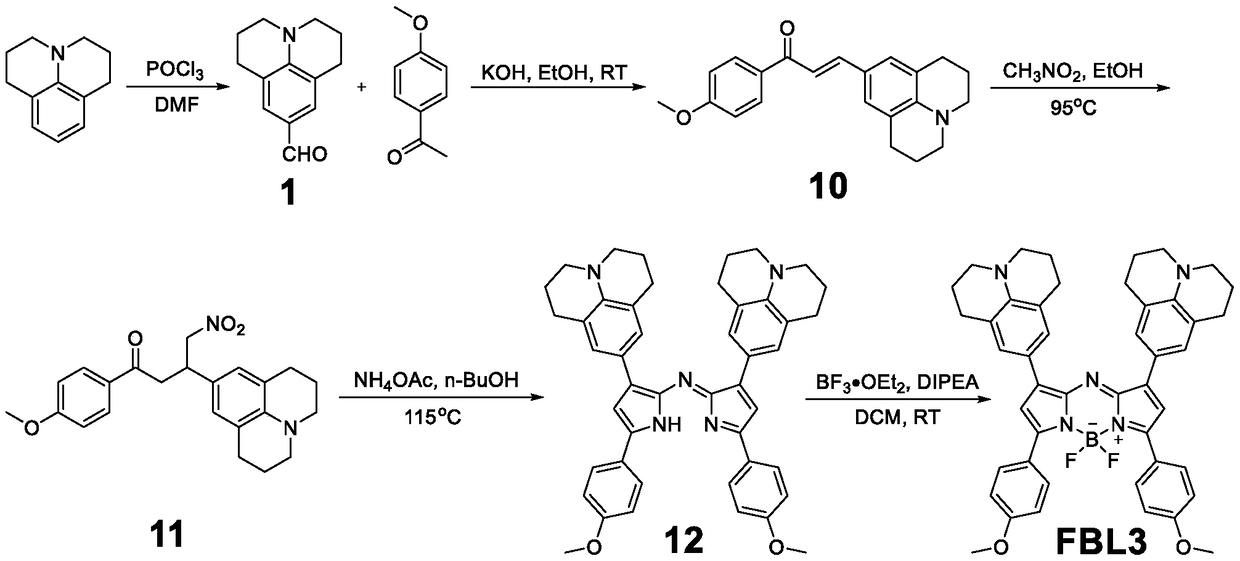
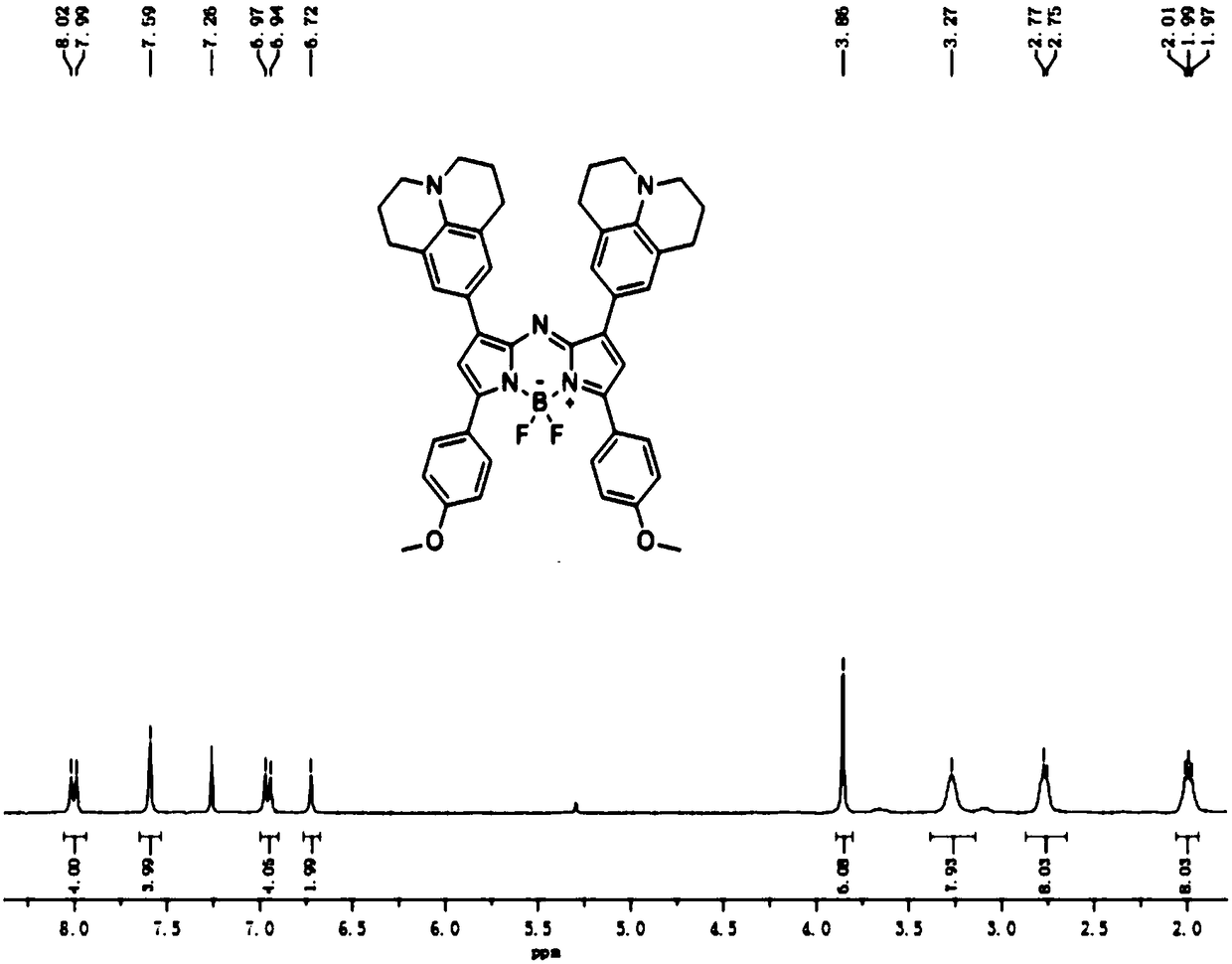
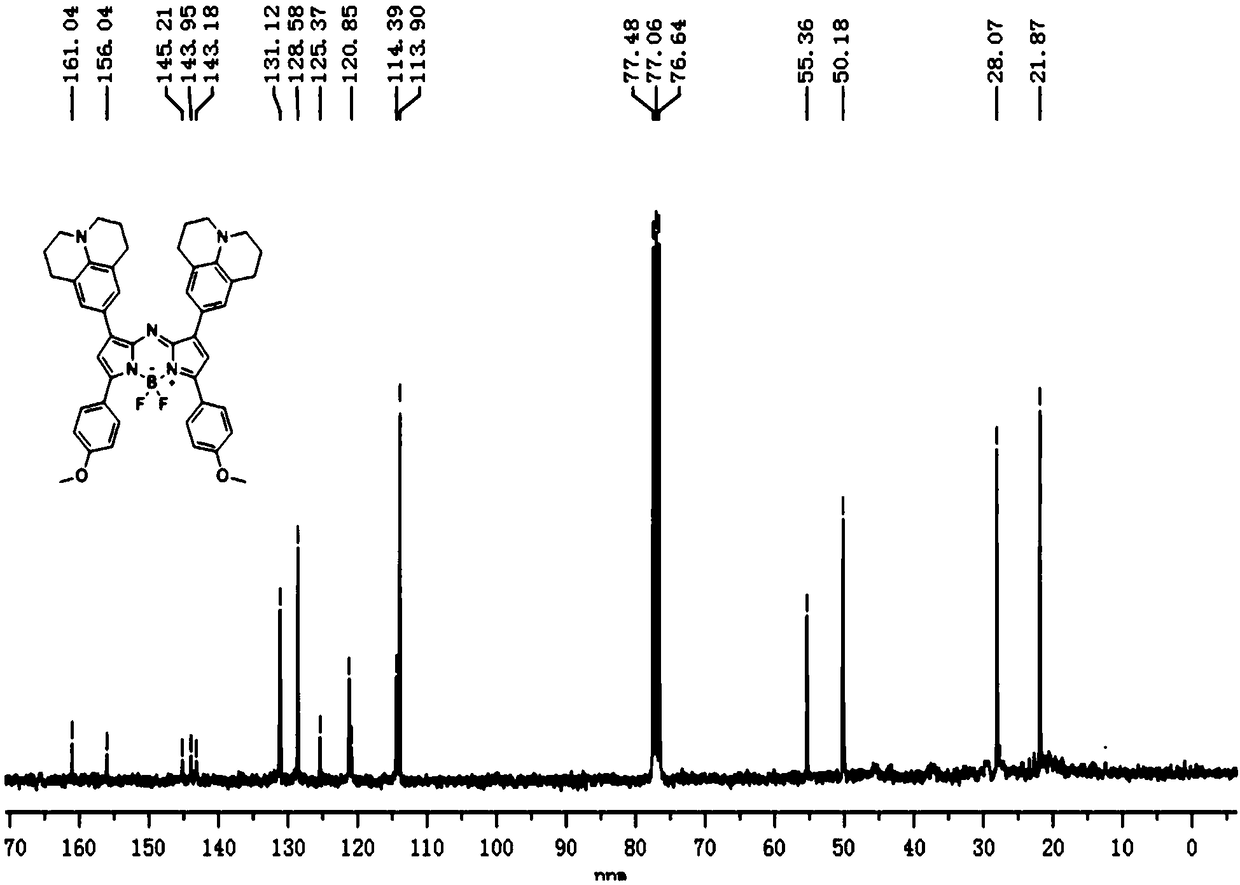
[0050] The preparation method of a series of Aza-BODIPY-based near-infrared two-window fluorescent probe FBL3 is as follows:
[0051] 1.1 1: Phosphorus oxychloride (1.17g, 7.62mmol) was slowly added dropwise to a round-bottomed flask containing DMF (1.67g, 22.87mmol) in an ice-salt bath, and the ice bath was removed after the addition was completed. Under the protection of nitrogen, continue stirring at room temperature for half an hour to prepare the Vilsmeier-Haack reagent. Then, the DMF solution dissolved with julonidine (1.2 g, 6.93 mmol) was slowly added dropwise thereto, and after the dropwise addition was completed, it was refluxed at 90° C. for 4 h. The reacted substance was poured into ice water to stop the reaction, and the stirring was continued for at least 2 h, a yellow solid was precipitated, and finally a light yellow solid 1 was obtained by suction filtration. 1 H NMR (300MHz, CDCl3) δ9.59(s,1H),7.28(s,2H),3.28(t,J=6.0,4H),2.76(t,J=6.0,4H),1.95(m,4H ). Its r...
Embodiment 2
[0060] The preparation method of a series of Aza-BODIPY-based near-infrared two-window fluorescent probe FBL3 is as follows:
[0061] 2.1 1: Phosphorus oxychloride (2.34g, 15.24mmol) was slowly added dropwise to a round-bottomed flask containing DMF (3.34g, 45.74mmol) in an ice-salt bath, and the ice bath was removed after the addition was completed. Under the protection of nitrogen, continue stirring at room temperature for half an hour to prepare the Vilsmeier-Haack reagent. Then, the DMF solution dissolved with julonidine (2 g, 11.55 mmol) was slowly added dropwise thereto, and after the dropwise addition was completed, it was refluxed at 90° C. for 4 h. The reacted substance was poured into ice water to stop the reaction, and the stirring was continued for at least 2 h, a yellow solid was precipitated, and finally a light yellow solid 1 was obtained by suction filtration.
[0062] 2.2 10: Add methoxyacetophenone (751mg, 5mmol), compound 1 (800mg, 3.94mmol), ethanol (60mL)...
Embodiment 3
[0066] The preparation method of the series based on Aza-BODIPY-based near-infrared two-window fluorescent probe FBL3 is as follows:
[0067] 3.1 1: Phosphorus oxychloride (0.585g, 3.81mmol) was slowly added dropwise to a round-bottomed flask containing DMF (1g, 13.72mmol) in an ice-salt bath, and the ice bath was removed after the addition was completed. Under nitrogen protection, continue stirring at room temperature for half an hour to prepare Vilsmeier-Haack reagent. Then a DMF solution in which julolidine (0.6 g, 3.46 mmol) was dissolved was slowly added dropwise thereto, and after the dropwise addition was completed, it was refluxed at 90° C. for 4 h. The reacted substance was poured into ice water to stop the reaction, and the stirring was continued for at least 2 h, a yellow solid was precipitated, and finally a light yellow solid 1 was obtained by suction filtration.
[0068]3.2 10: Add methoxyacetophenone (375.5mg, 2.5mmol), compound 1 (300mg, 1.476mmol), ethanol (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com