Platinum-based bodipy compound, preparation method and application
A technology for fluorinated boron dipyrrole and compound, which is applied to platinum-based fluorinated boron dipyrrole compounds and the fields of preparation and application, and can solve the problem of limited application of antitumor drugs, insignificant solubilization effect, easy quenching of singlet oxygen, etc. problem, to achieve the effect of improving photodynamic therapy activity, enhancing non-radiative transition, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Prepare R 1 Be the platinum-based fluoroboron dipyrrole compound of polyethylene glycol group (molecular weight is 5000), concrete steps are as follows:
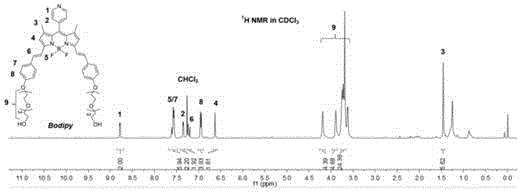
[0046] (1) Preparation of fluorobodipyrrole derivatives: 2,4-dimethylpyrrole, p-formylpyridine, 2,3-dichloro Add -5,6-dicyano-p-benzoquinone, diisopropylethylamine, boron trifluoride ethyl ether, and trifluoroacetic acid into 50mL of dichloromethane, and react at 25°C (room temperature) for 16h to obtain fluoroboron dipyrrole derivatives thing;
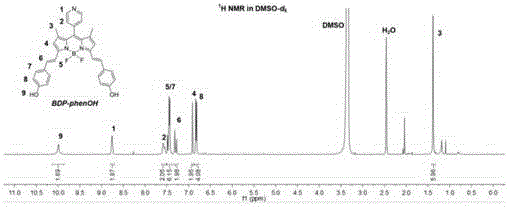
[0047] (2) Preparation of fluorobodipyrrole conjugates: Dissolve 130 mg of fluorobodipyrrole derivatives obtained in step (1) and 147 mg p-hydroxybenzaldehyde in 50 ml of toluene, and add 1 mL of glacial acetic acid and 1 mL of piperidine , heated to reflux at 112°C for 20 hours under a nitrogen atmosphere, and ice-bathed for 30 minutes. The resulting solid product was washed several times with toluene, and then spin-dried by a rotary evaporator to obtain the primary produc...
Embodiment 2
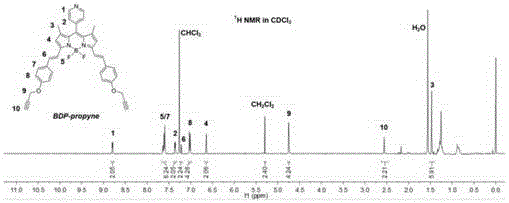
[0054] The steps of this example are basically the same as those of Example 1, the difference being that in step (3), 80 mg of fluoroborate dipyrrole conjugates and 207 mg of 3-bromopropyne are added to 50 mL of acetonitrile, and potassium carbonate is used as a catalyst, heated and condensed to reflux After 24 hours of reaction, the product yield was 30%.
Embodiment 3
[0056] The steps of this example are basically the same as those of Example 1, the difference being that in step (5), under light-shielding conditions, 10 mg of platinum-based fluoroborate dipyrrole derivatives containing alkynyl groups at the terminal, 20 mg of alkynyl dipyrrole derivatives with a molecular weight of 1000, and Nitrogen polyethylene glycol polymer (molar ratio 1:2) is added to 10% of anhydrous and oxygen-free dimethyl sulfoxide solvent, and an appropriate amount of copper sulfate pentahydrate and sodium ascorbate are added as catalysts, operated in a glove box, 50 ° C After 24 hours of reaction, the final product yield was 30%.
[0057] The platinum-based fluoroboron dipyrrole compound prepared in this example is prepared for the preparation of nanomicelles. The specific steps are: weigh 1 mg of the sample, dissolve it in 5 mL of dichloromethane, and remove the dichloromethane by rotary evaporation with a rotary evaporator, so that The substance forms a thin f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com