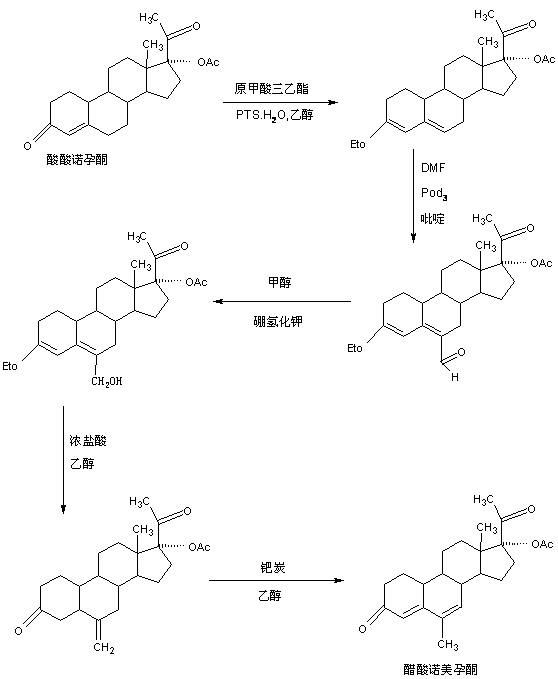
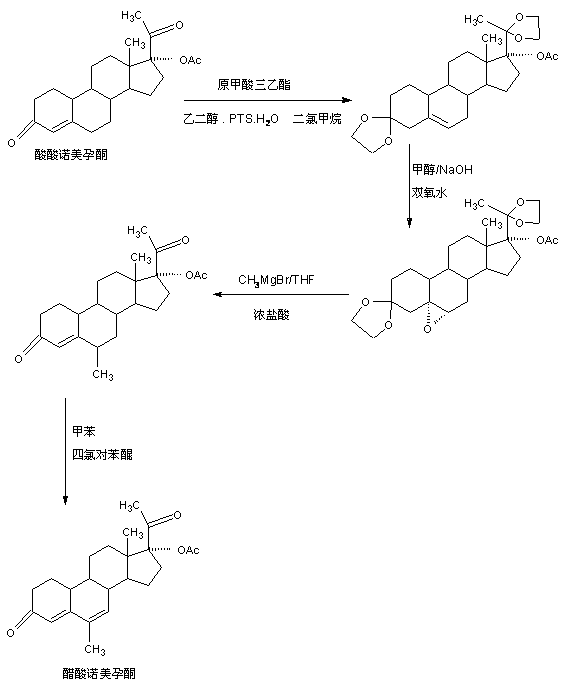
The preparation method of nomegestrol acetate
A technology of nomegestrol acetate and norgegestrel acetate, which is applied in the field of preparation of steroid hormone drugs, can solve the problems of low rearrangement reaction yield, harsh reaction conditions, high production cost, etc., and achieve low production cost and comprehensive synthetic route The effect of shortening and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A, the preparation of bisketal
[0034] In a 1000ml three-neck flask, add 100g norgestrel acetate, 600ml dichloromethane, 50g ethylene glycol, 80ml triethyl orthoformate, 2g p-toluenesulfonic acid, keep warm at 25-30°C and stir for 12-16 hours. TLC detects the end point of the reaction. After the reaction, add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then concentrate under reduced pressure to recover 90-95% of dichloromethane, then add 600ml of tap water, stir and crystallize at 10-15°C After 3-4 hours, centrifuge and wash with water to obtain the crude product of bisketal. The above-mentioned crude product is not baked, directly added to 800ml of 50% alcohol, first refluxed for 1-1.5 hours, and then about 400ml of alcohol is evaporated under normal pressure, and then The system was cooled to -5-0°C, stirred and crystallized for 2-3 hours, suction filtered, washed with a small amount of ethanol, and the filter cake was dried below 70°C to obtain 1...
Embodiment 2
[0042] A, the preparation of bisketal
[0043] In a 1000ml three-neck flask, add 100g norgestimate acetate, 600ml toluene, 50g ethylene glycol, 80ml triethyl orthoformate, 2g 98% sulfuric acid, keep warm at 25-30°C and stir for 12-16 hours, TLC detection At the end of the reaction, after the reaction, add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then concentrate under reduced pressure, recover 90-95% of toluene, then add 600ml of tap water, stir and crystallize at 10-15°C for 3-4 hours , centrifuged and washed with water to obtain the crude product of bisketal. The above crude product is not baked, directly added to 800ml of 50% alcohol, first refluxed for 1-1.5 hours, then steamed out about 400ml of alcohol at normal pressure, then cooled the system to -5-0°C, stirred and crystallized for 2-3 hours, Suction filtration, washing with a small amount of ethanol, and drying of the filter cake below 70°C yielded 116.8 g of bisketal product with an HPLC conte...
Embodiment 3
[0051] A, the preparation of bisketal
[0052] In a 1000ml three-necked flask, add 100g norgestrel acetate, 600ml dichloromethane, 50g ethylene glycol, 80ml triethyl orthoformate, 10g ethanol solution of hydrochloric acid, keep warm at 25~30℃ and stir for 12~16 After hours, TLC detects the end of the reaction. After the reaction, add 3ml of pyridine, stir for 20-25 minutes to neutralize the acid, then concentrate under reduced pressure to recover 90-95% of dichloromethane, then add 600ml of tap water, and stir at 10-15°C Crystallize for 3-4 hours, centrifuge and wash with water to obtain the crude product of bisketal. The above crude product is not baked, directly added to 800ml of 50% alcohol, first refluxed for 1-1.5 hours, then steamed out about 400ml of alcohol at normal pressure, then cooled the system to -5-0°C, stirred and crystallized for 2-3 hours, pumped Filter, wash with a small amount of ethanol, and dry the filter cake below 70°C to obtain 116.2 g of bisketal, wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com