Bridged bis-boron-dipyrromethene (BODIPY) derivative containing fluorene at meso-position and preparation method thereof
A technology of dipyrromethene and boron bisfluoride, applied in the field of boron bisfluoride complexed dipyrromethene derivatives, achieving the effect of high comprehensive yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
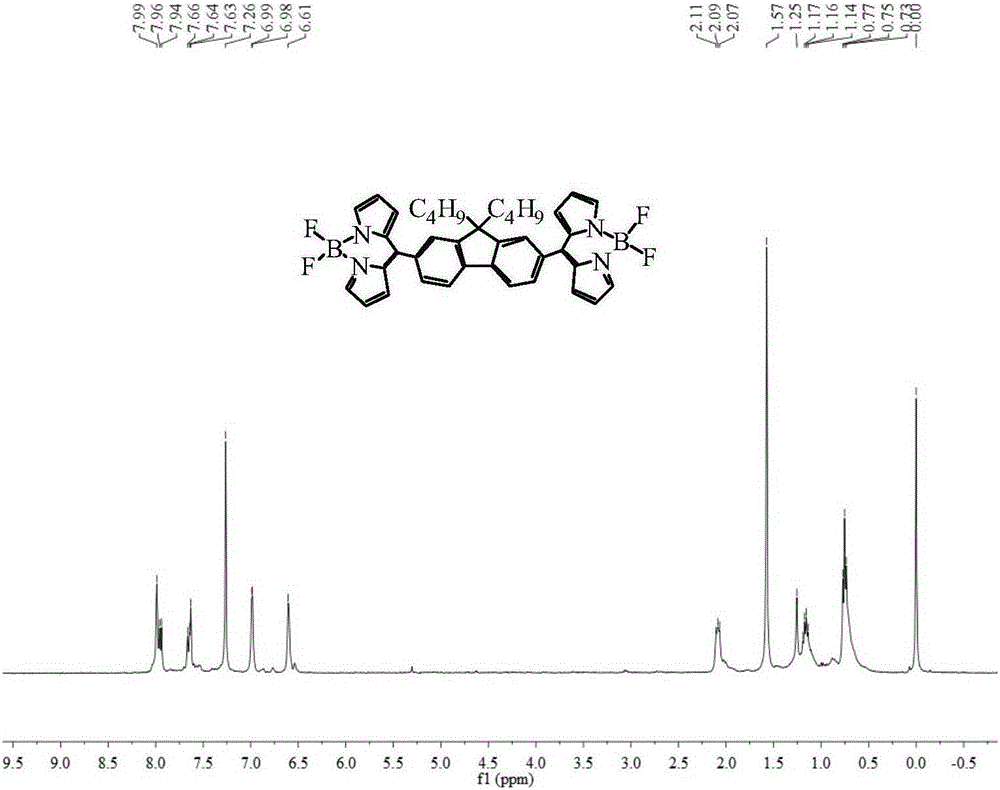
[0080] Synthesis of BDP1
[0081] Add vacuum-dried 9,9'-dibutyl-2,7-dipyrromethene fluorene (285mg, 0.5mmol) and tetrachlorobenzoquinone (250mg, about 1mmol) to a 100ml three-necked flask, in dichloromethane The solution was oxidized with magnetic stirring at room temperature for 8 hours, and the solution in the three-neck flask changed from dark yellow to dark red. Then put the reaction on N 2 Under the protective device, boron trifluoride diethyl ether (4mL, 28mmol) was slowly added dropwise, and triethylamine (4mL, 28mmol) was added in portions after 5min, and the reaction was continued for 4h, and the reaction was stopped. Wash with anhydrous ether and water successively, and dry the organic phase with anhydrous magnesium sulfate. The solvent was spun off under reduced pressure, and the crude product was subjected to silica gel column chromatography with a developer of dichloromethane:petroleum ether=1:1 to obtain 278 mg of a bright yellow solid with a yield of 87% and a...
Embodiment 2
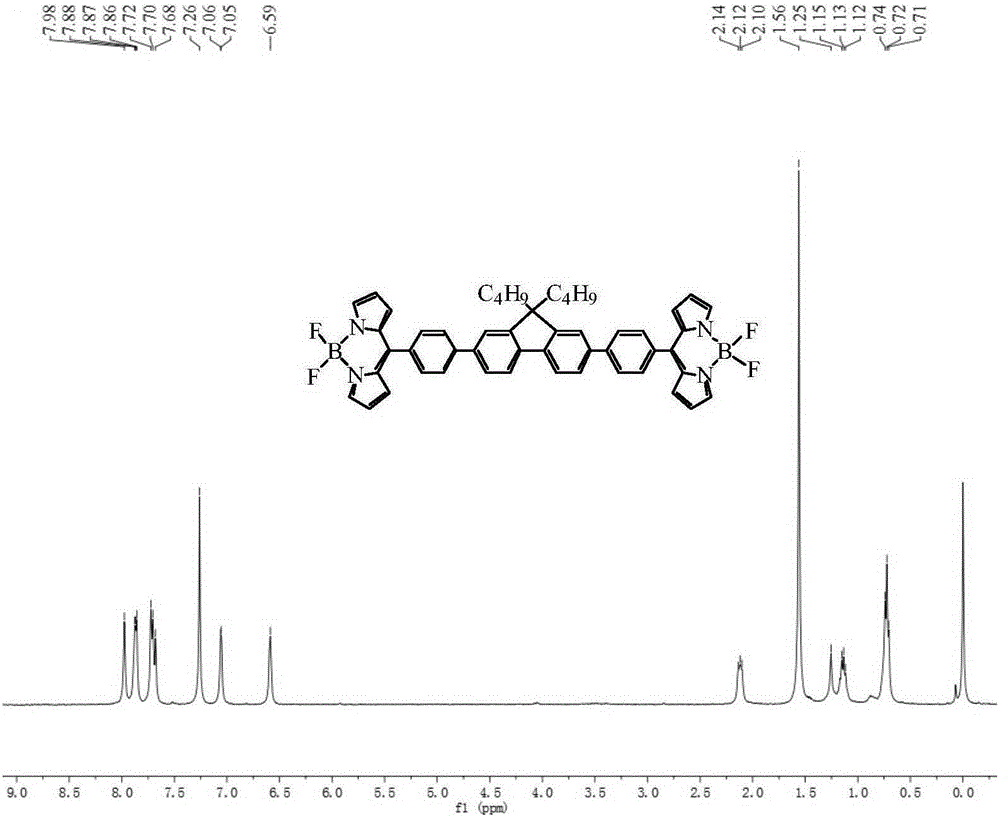
[0083] Synthesis of BDP2
[0084] Using a method similar to the synthesis of BDP1, 2,7-bis[4-(dipyrrolemethyl)phenyl]-9,9-dibutylfluorene was oxidized by TCQ, and reacted with boron trifluoride ether to obtain the target product BDP2, 82% yield, melting point >320°C. 1 HNMR (400MHz, CDCl 3 ,TMS,ppm):δ:7.98(s,4H),7.88-7.86(m,6H),7.68-7.72(m,8H),7.05-7.06(d,J=2.7Hz,4H),6.59(s ,4H),2.10-2.14(t,J=6.8Hz,4H),1.12-1.15(m,8H),0.71-0.74(t,J=7.2Hz,6H); 13 CNMR (100MHz, CDCl 3,TMS,ppm):δ:150.70,147.61,144.16,144.07,138.92,134.56,131.57,131.49,131.26,127.16,126.36,121.54,120.53,118.56,55.23,40.30,29.70,26.13,23.08,13.83.MALDI- TOF-MS, m / z:calcdforC 51 h 44 B 2 f 4 N 4 [M-F] + :791.409,found:791.246.
Embodiment 3
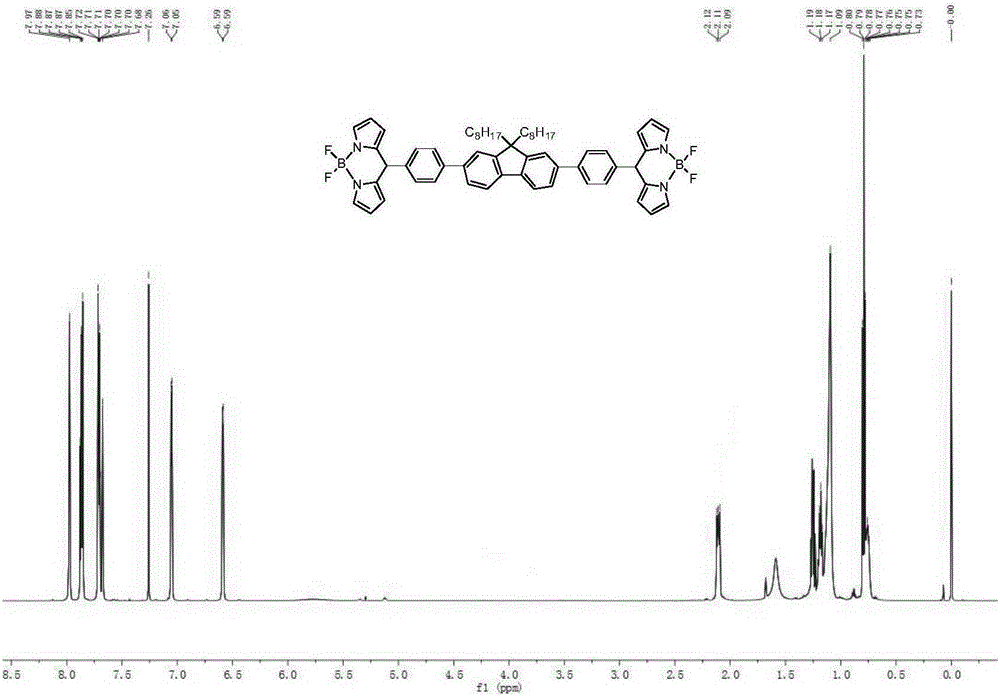
[0086] Synthesis of BDP3
[0087] According to the method of synthesizing BDP2, the compound 2,7-bis[4-(dipyrrolemethyl)phenyl]-9,9-dioctylfluorene (0.5g, 0.6mmol), tetrachloro Benzoquinone and dry dichloromethane were fully oxidized for 6 hours with magnetic stirring at room temperature. Then exhaust the air in the reaction system, pass it into argon protection, slowly add triethylamine dropwise through a syringe, and then use a syringe to take boron trifluoride ether and add it dropwise. The drop rate of boron diethyl ether, after the dropwise addition, continued the magnetic stirring reaction for 4h. The reaction was stopped, and the reaction mixture was poured into NaOH aqueous solution (200 mL, 0.1 M), extracted several times with dichloromethane, washed with water three times, and the organic phases were combined and dried overnight over anhydrous sodium sulfate. The filtrate was collected by filtration, the solvent was evaporated under reduced pressure, and the residu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com