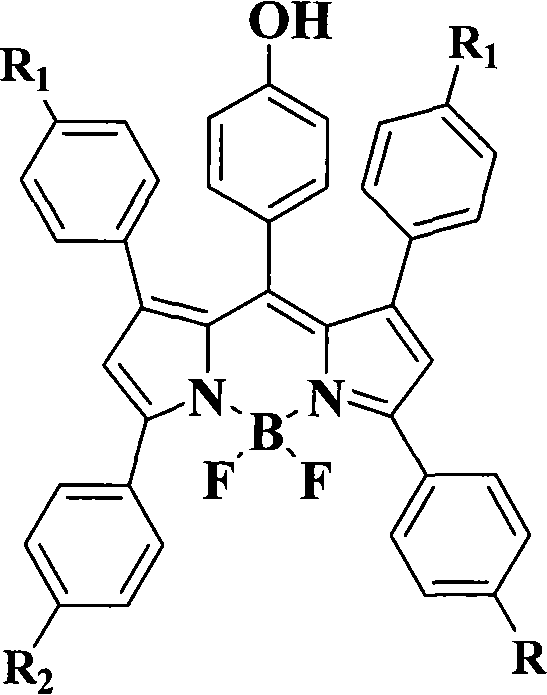
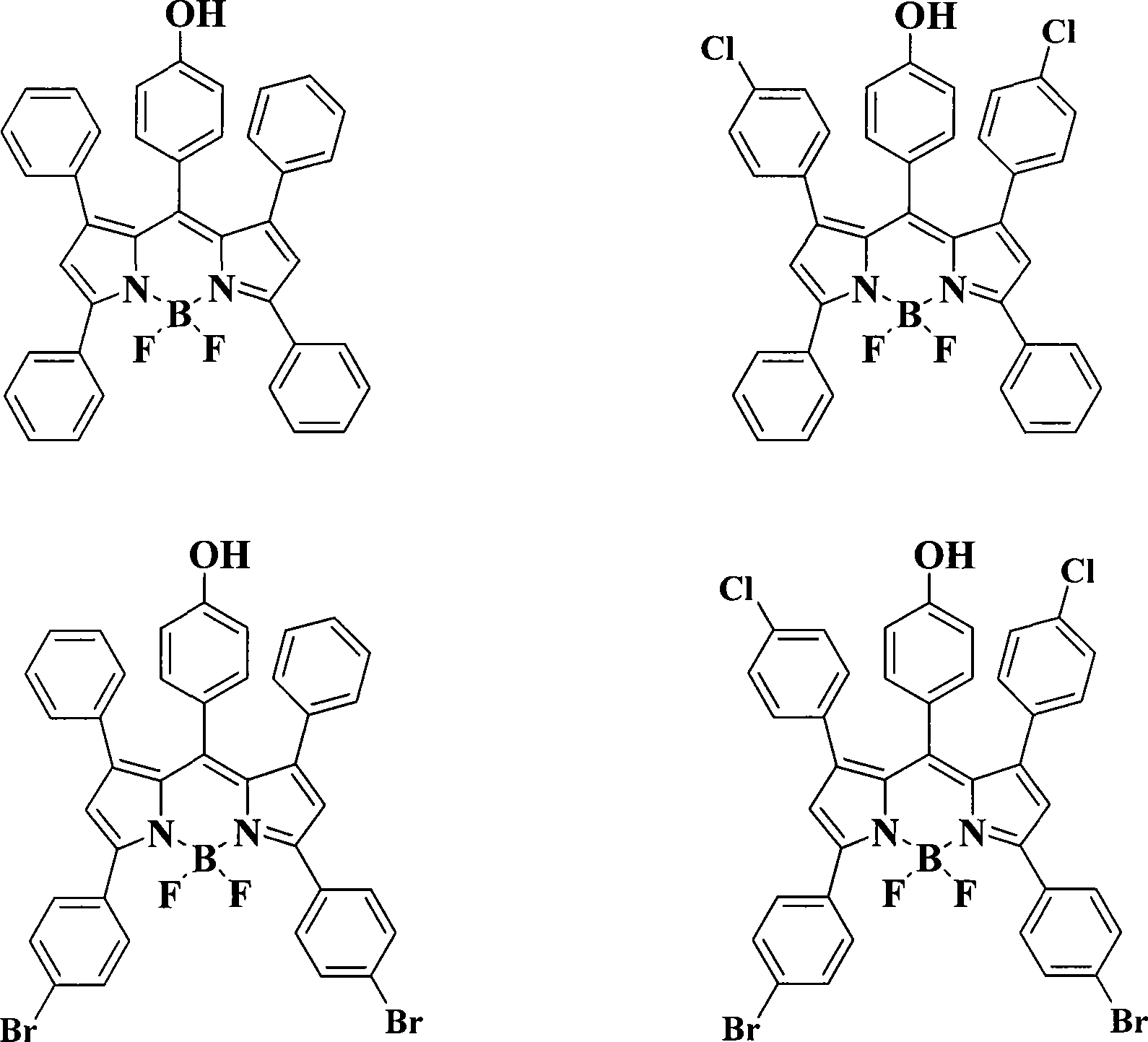
Pyrrole dimethine fluorescent dyes as well as synthetic method and use thereof
A technology for pyrrole dimethacrylate and fluorescent dyes, which is applied in the field of synthesis of the fluorescent dyes and achieves the effects of good chemical stability and photostability, and suitable excitation and emission wavelengths.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] a. Dissolve styrene and liquid bromine in carbon tetrachloride respectively, and slowly add the carbon tetrachloride solution with liquid bromine into the carbon tetrachloride solution with styrene at 15°C, and stir for 5 After reaction finishes, carbon tetrachloride is distilled off under reduced pressure, obtains white crystal 1,2-dibromostyrene, and described white crystal 1,2-dibromostyrene is dissolved in dimethyl sulfoxide, Slowly add sodium azide under nitrogen protection, stir at 20°C for 24 hours, then add aqueous sodium hydroxide solution to make it in a strong alkaline environment, continue stirring for 36 hours, and then add the reaction product to the sodium bicarbonate solution, and use Dichloromethane extraction, the organic layer was washed with water, and the organic solvent was distilled off under reduced pressure to obtain a dark red oil, which was separated on a silica gel column and eluted with petroleum ether as an eluent, and then evaporated under ...
Embodiment 2
[0033] a. Dissolve styrene and liquid bromine in carbon tetrachloride respectively, and slowly add the carbon tetrachloride solution with liquid bromine into the carbon tetrachloride solution with styrene at 20°C, and stir for 1 After reaction finishes, carbon tetrachloride is distilled off under reduced pressure, obtains white crystal 1,2-dibromostyrene, and described white crystal 1,2-dibromostyrene is dissolved in dimethyl sulfoxide, Slowly add sodium azide under the protection of argon, stir at 28°C for 10 hours, then add aqueous sodium hydroxide solution to make it in a strong alkaline environment, continue stirring for 12 hours, then add the reaction product to sodium bicarbonate solution, and use Dichloromethane extraction, the organic layer was washed with water, and the organic solvent was distilled off under reduced pressure to obtain a dark red oil, which was separated on a silica gel column and eluted with petroleum ether as an eluent, and then evaporated under redu...
Embodiment 3
[0038] Take 5.2 grams (0.05mol) of styrene and dissolve it in carbon tetrachloride, and dissolve 8.0 grams (0.10mol) of liquid bromine in carbon tetrachloride. Keep the temperature in an ice-water bath at 15° C. The carbon solution was slowly added to the carbon tetrachloride solution dissolved in styrene, controlled at 15°C, and stirred for 5 hours. After completion of the reaction, carbon tetrachloride was removed by distillation under reduced pressure to obtain white crystal 1,2-dibromostyrene, and the white crystal 1,2-dibromostyrene was dissolved in dimethyl sulfoxide. Slowly add 4.9 grams (0.075mol) of sodium azide under air protection, keep the temperature at 28°C and continue to stir for 10 hours, then add a certain volume of aqueous solution containing 2.0 grams of sodium hydroxide to the mixture to make it in a strongly alkaline environment, continue After stirring for 12 hours, the reaction product was poured into a certain volume of 2% sodium bicarbonate solution, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com