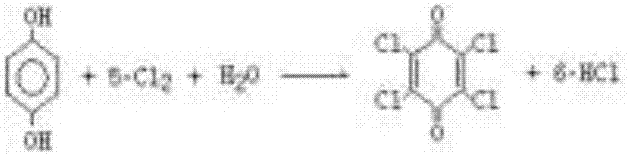
Preparation method of chloranil
A technology of chloranil and chlorine gas, which is applied in the field of dye intermediates, can solve the problems of high cost and high price of chloranil, and achieve the effects of environmental friendliness, low comprehensive cost and good economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation method of chlorobenzoquinone comprises the following steps: in the first chlorine tank, stirring and dissolving p-aminophenol into the acetic acid solution, the mass ratio of acetic acid in the acetic acid solution is 20%, the mass ratio of p-aminophenol to the acetic acid solution The ratio is 10:25, and then chlorine gas is introduced, and the reaction is initially carried out at 55° C., and the temperature will gradually increase when the chlorine is passed. The crystallization of chloranil is obviously good, and the crystals of chloranil are obtained, and the ammonium chloride also begins to crystallize, and the hydrogen chloride gas produced in the reaction is absorbed by water to become hydrochloric acid.
[0020] Chlorine gas needs to be fed into the following mass: the molar ratio of p-aminophenol to chlorine gas is 1:5.5;
[0021] Iron is added to the acetic acid solution as a catalyst, and the iron is metallic iron. The mass of iron element in...
Embodiment 2
[0024] The preparation method of chlorobenzoquinone comprises the following steps: in the first chlorine tank, p-aminophenol is stirred and dissolved in the acetic acid solution, the mass ratio of acetic acid in the acetic acid solution is 30%, and the mass ratio of p-aminophenol to the acetic acid solution It is 10:75, then feed chlorine gas, react at 90°C, stop feeding chlorine when the yellow crystals of chlorobenzoquinone in the detection material obviously appear, and obtain chlorobenzoquinone crystals, ammonium chloride crystals, and the hydrogen chloride produced in the reaction The gas is absorbed by water to become hydrochloric acid. After the acetic acid solution is saturated, a pipeline is passed from the gas outlet of the first chlorine-passing tank into the acetic acid solution in the second chlorine-passing tank.
[0025] After the acetic acid solution is saturated, connect a pipeline from the gas outlet of the first chlorine tank to the acetic acid solution in t...
Embodiment 3
[0028] The preparation method of chlorobenzoquinone comprises the following steps: in the first chlorine tank, p-aminophenol is stirred and dissolved in the acetic acid solution, the mass ratio of acetic acid in the acetic acid solution is 30%, and the mass ratio of p-aminophenol to the acetic acid solution It is 10: 100, feeds chlorine gas then, reacts at 35 DEG C, can gradually heat up in the process of passing chlorine, but also can not exceed 90 DEG C, stops feeding chlorine when the chlorobenzoquinone obviously appears yellow crystal in the detection material, obtains Chlorobenzoquinone crystals, ammonium chloride crystals, hydrogen chloride gas generated during the reaction is absorbed by water to become hydrochloric acid. After the acetic acid solution is saturated, a pipeline is passed from the gas outlet of the first chlorine-passing tank into the acetic acid solution in the second chlorine-passing tank.
[0029] After the acetic acid solution is saturated, the gas ou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com