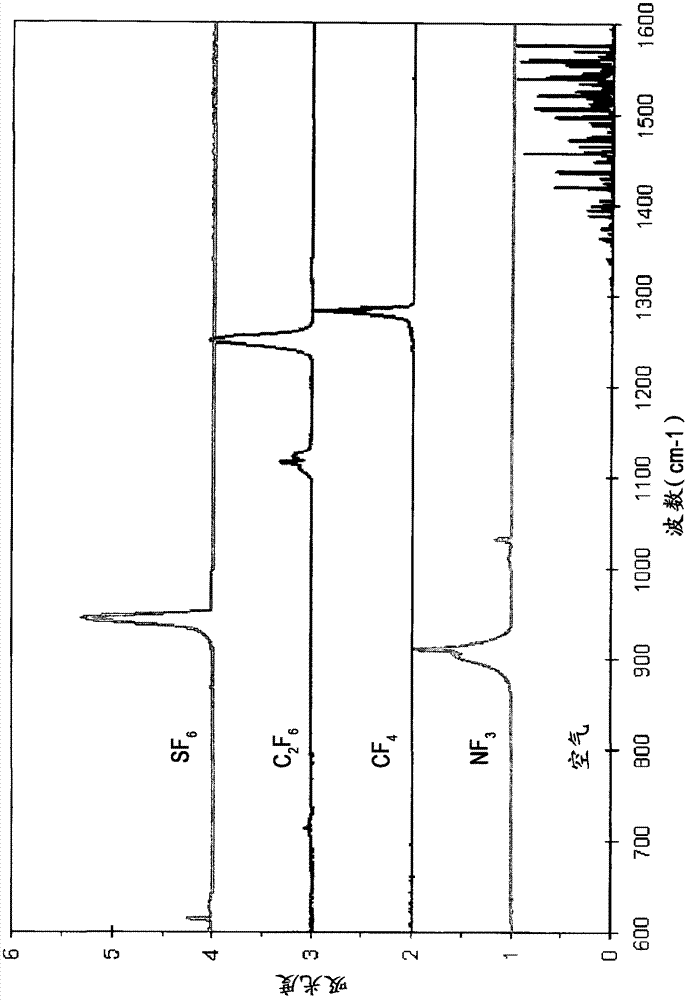
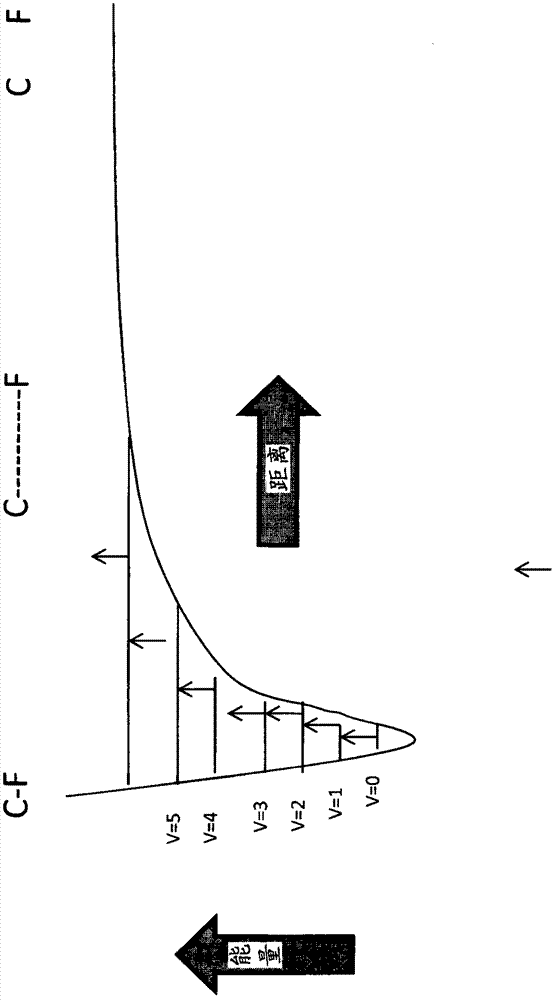
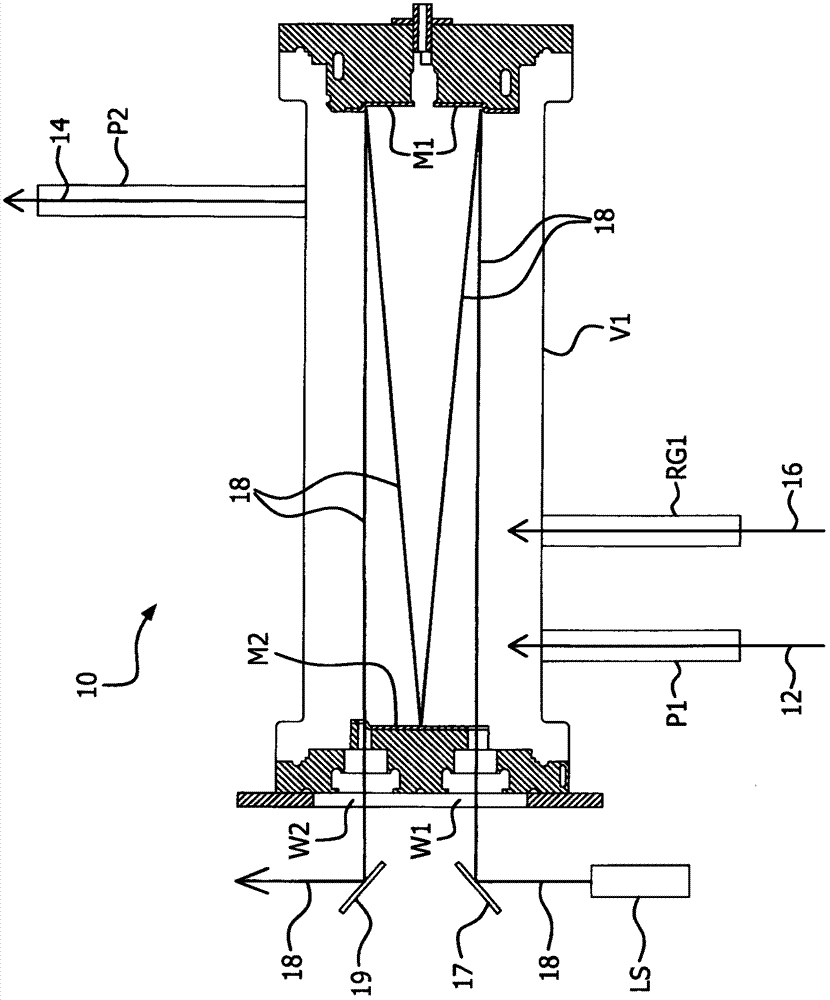
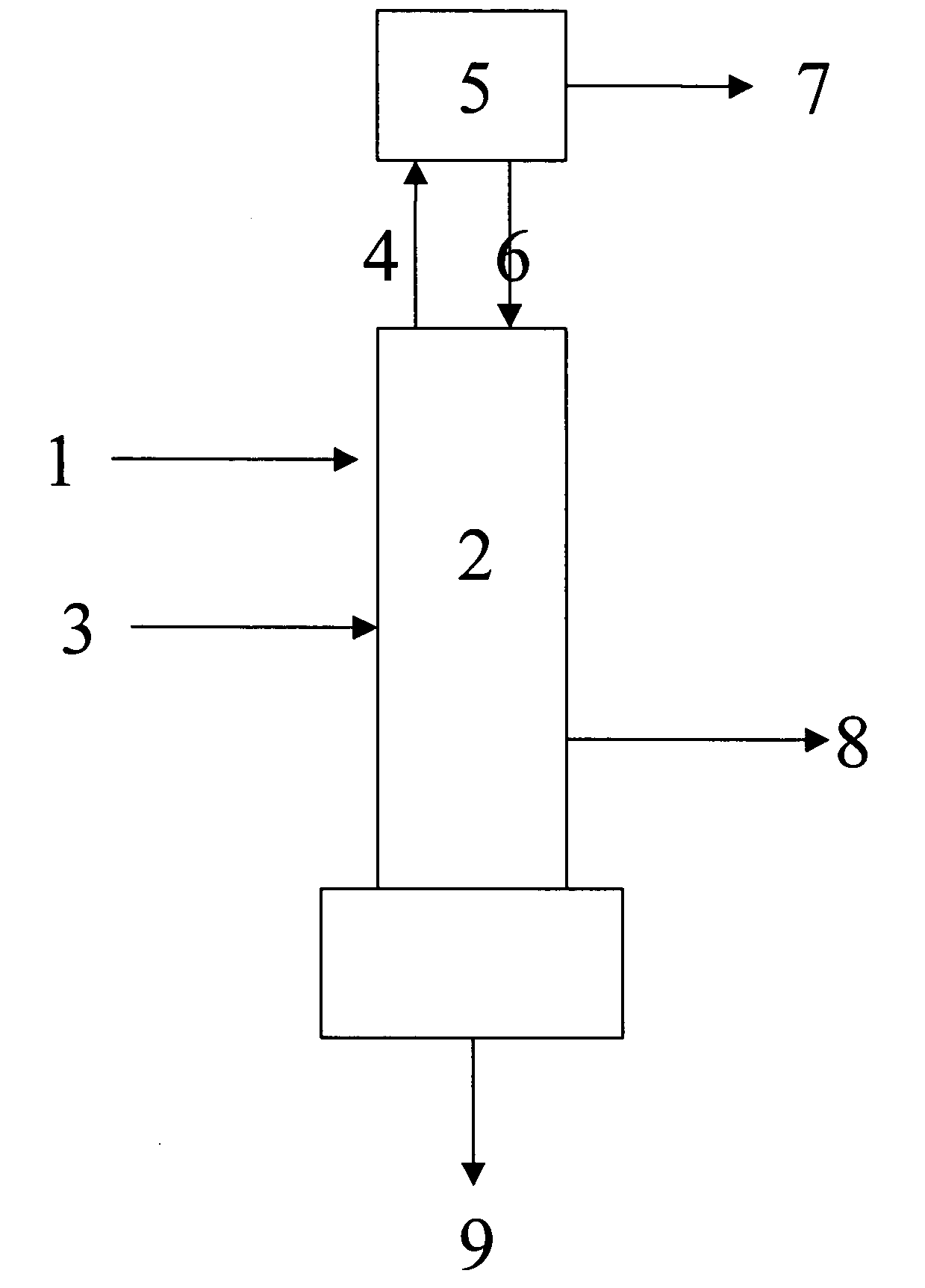
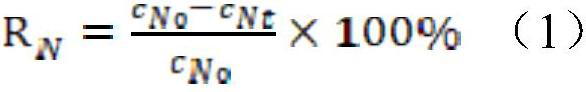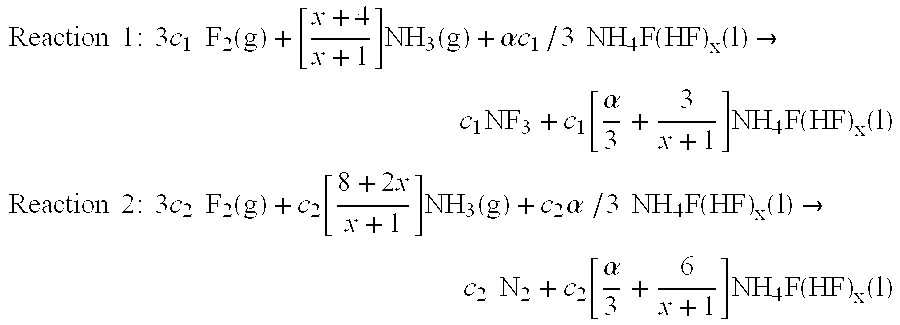Patents
Literature
40results about "Nitrogen trifluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
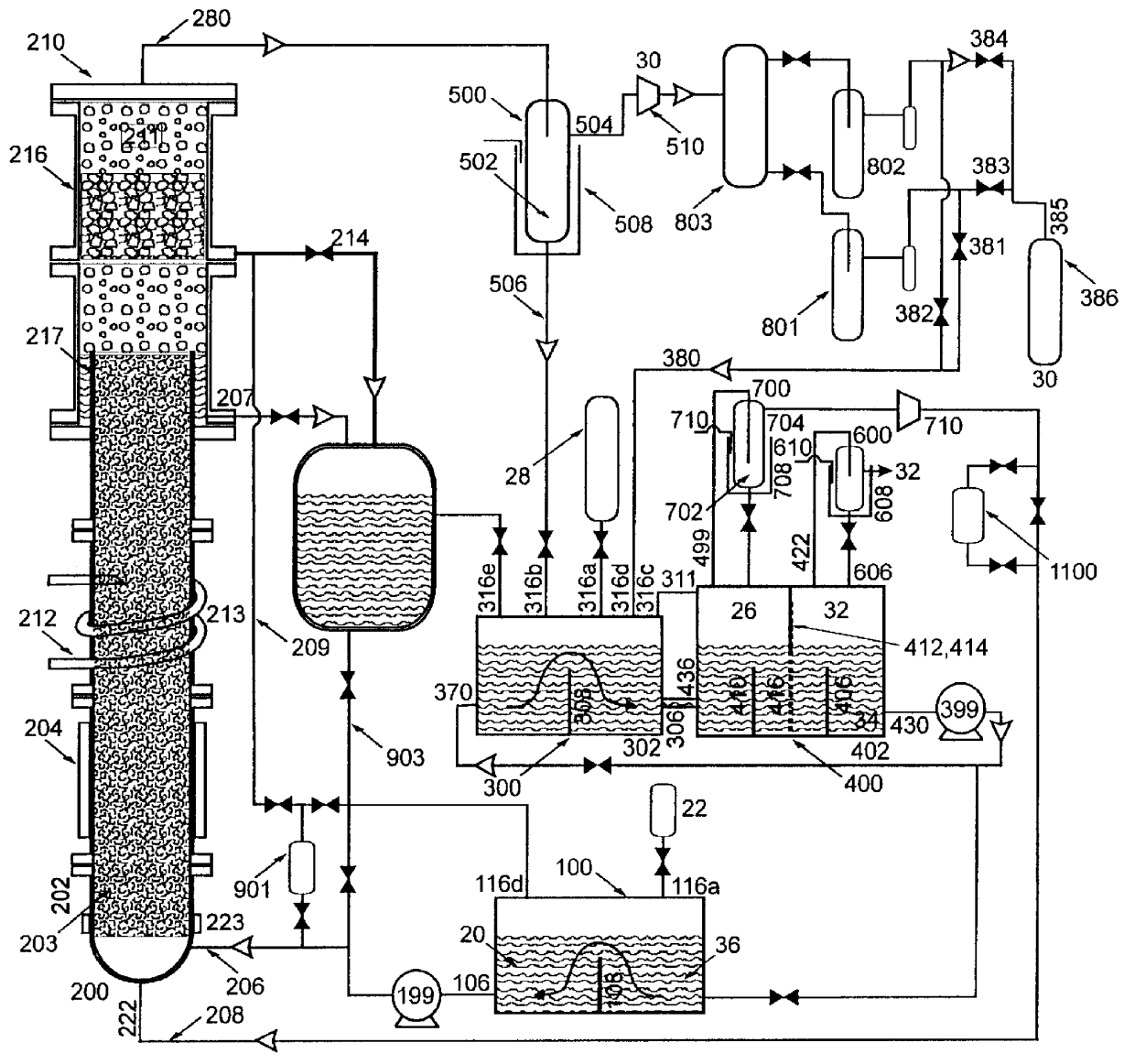
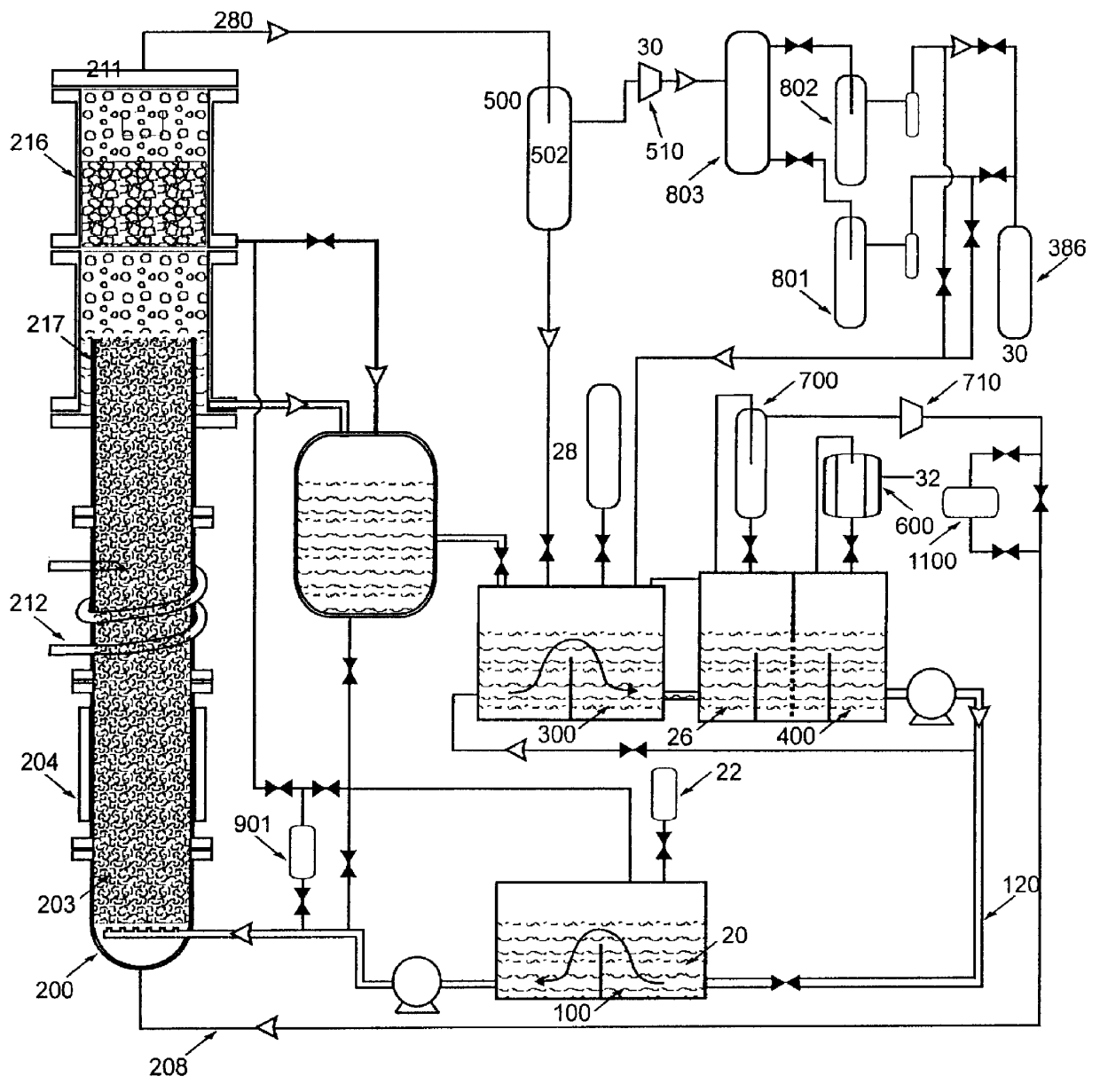
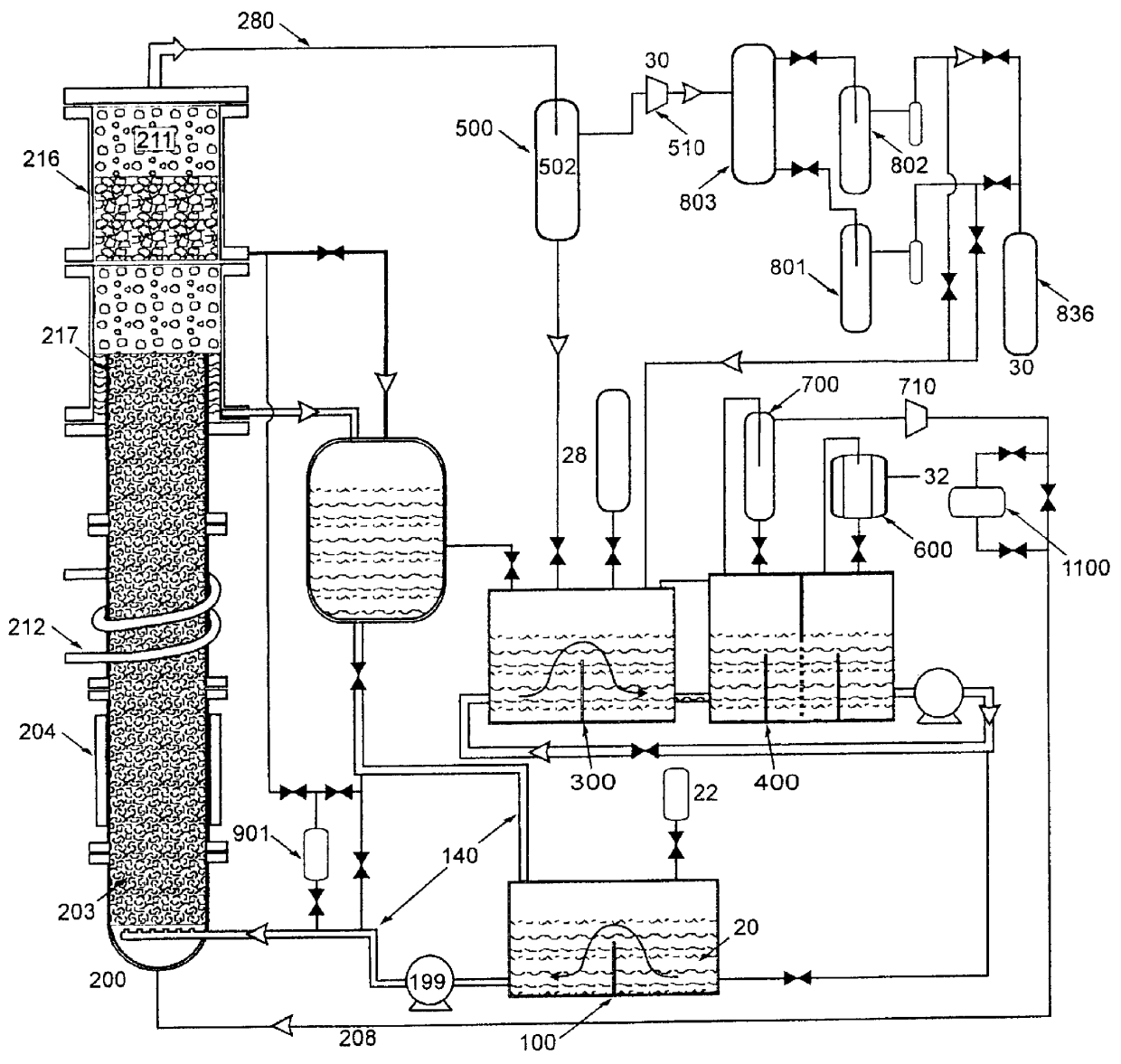
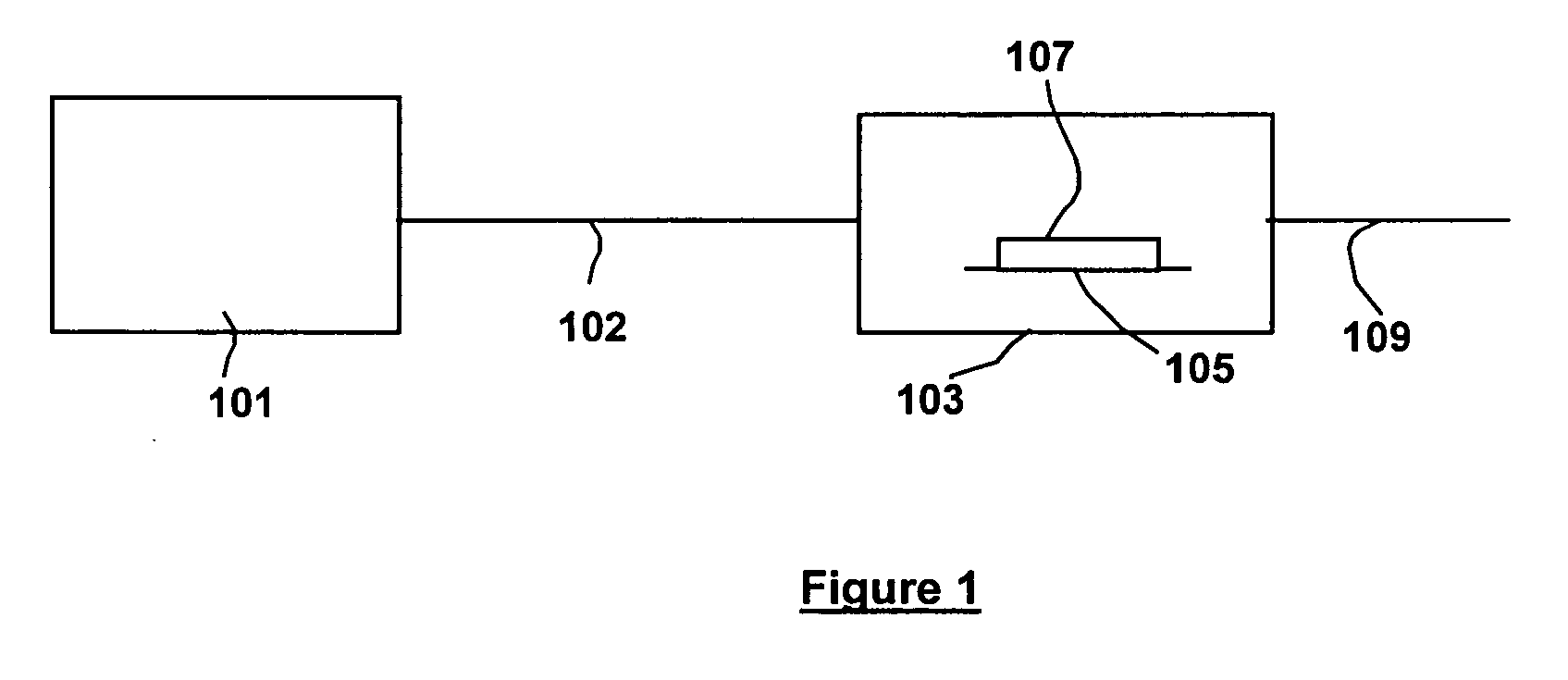
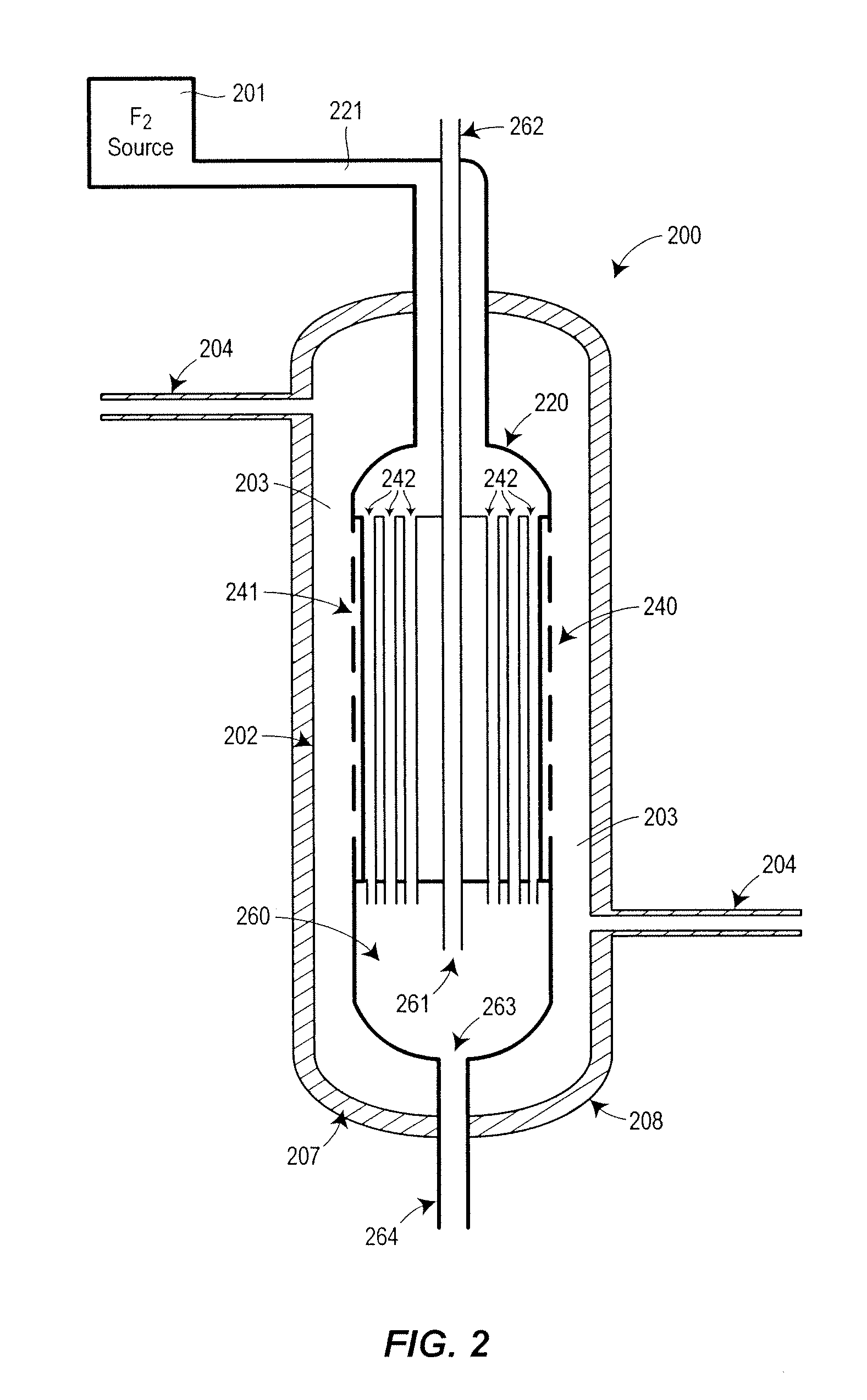
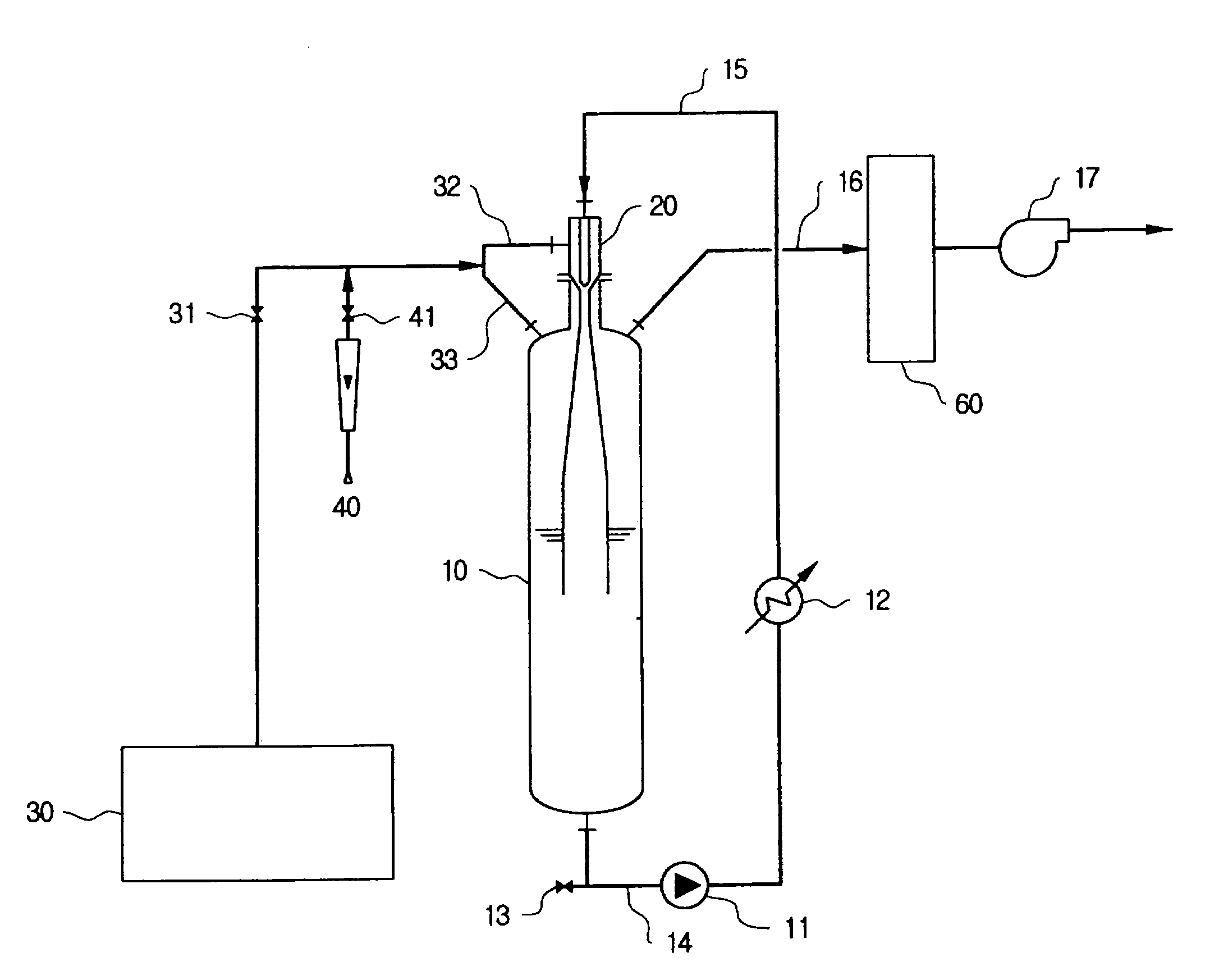
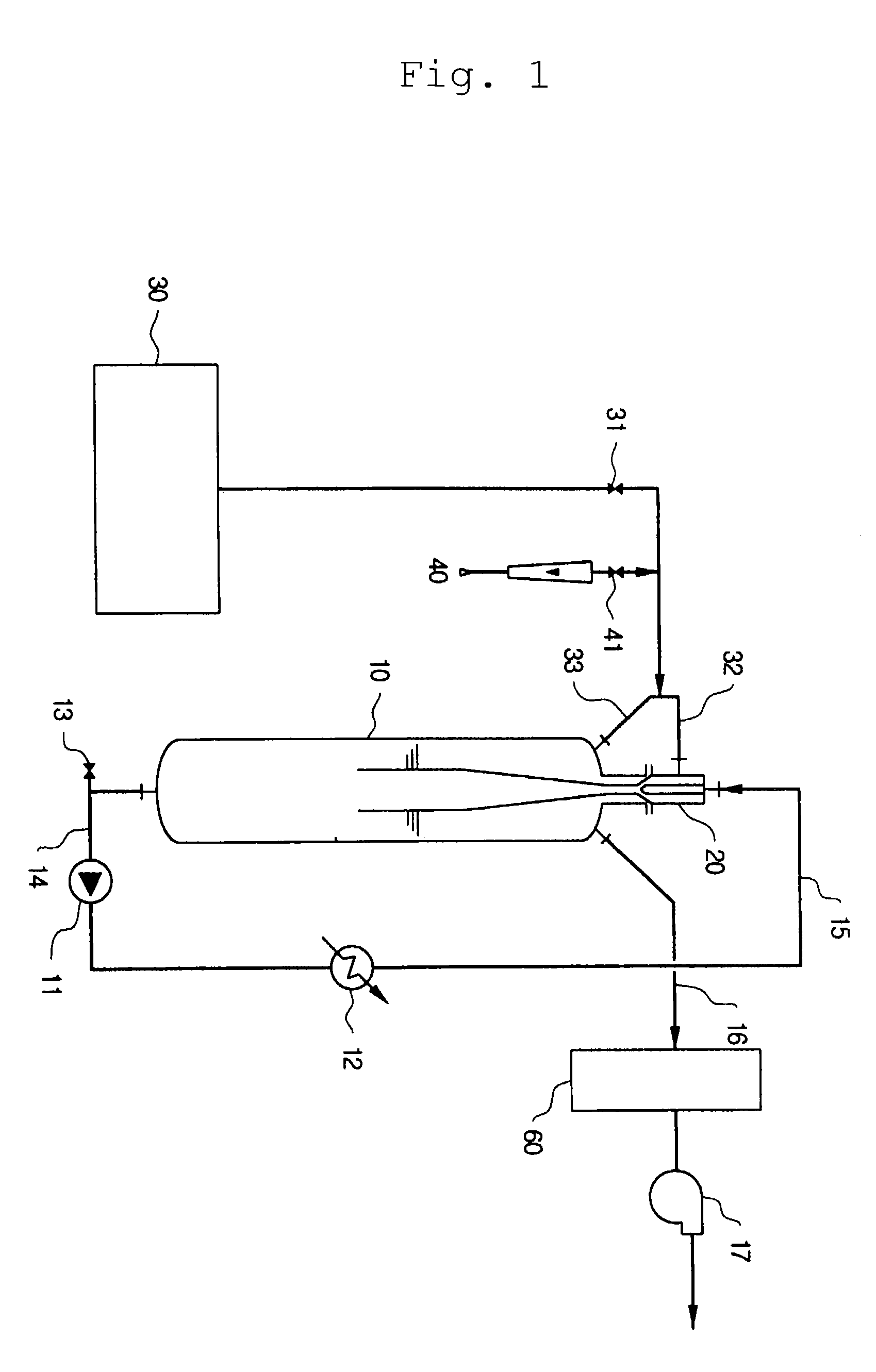
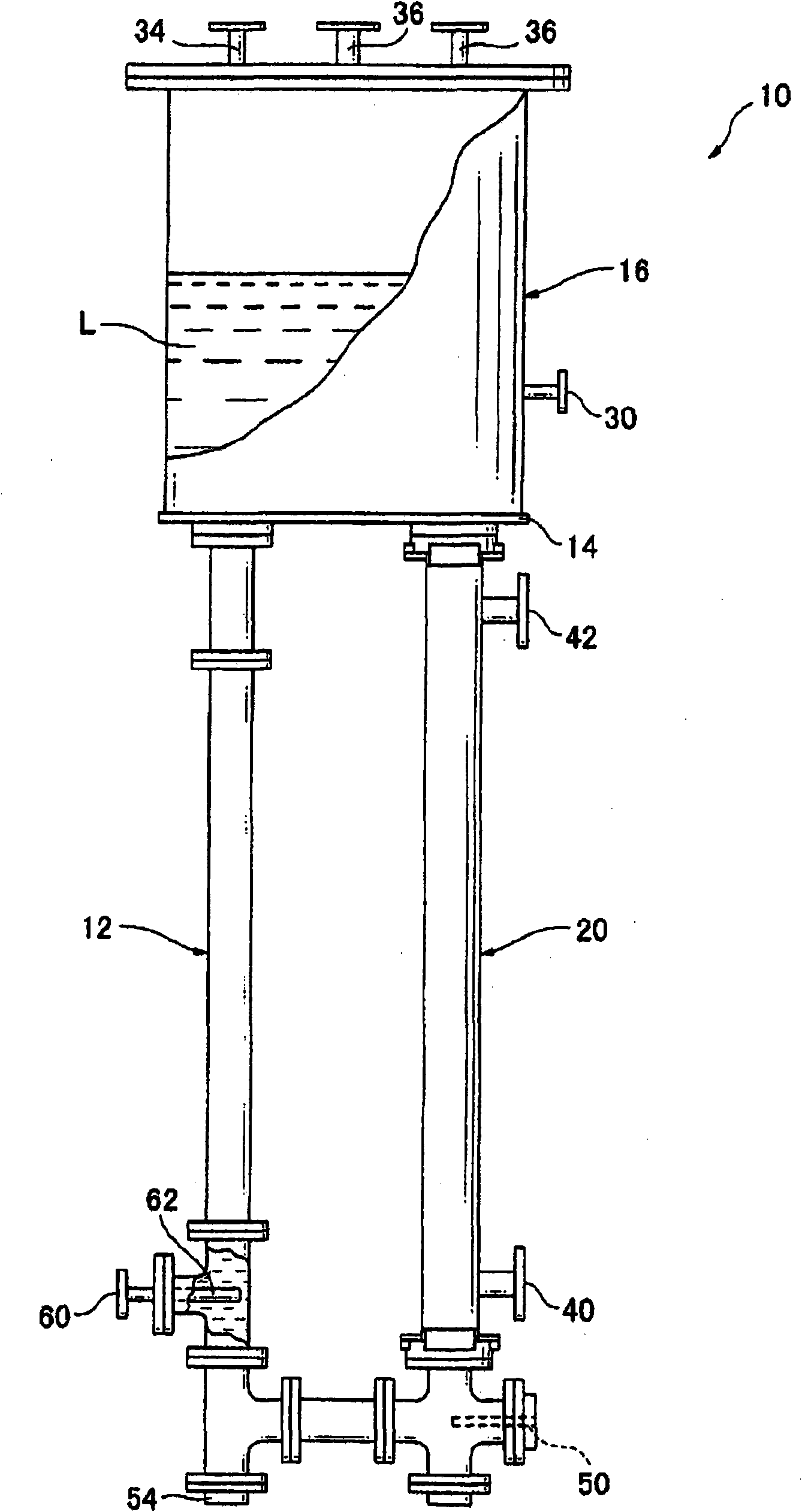
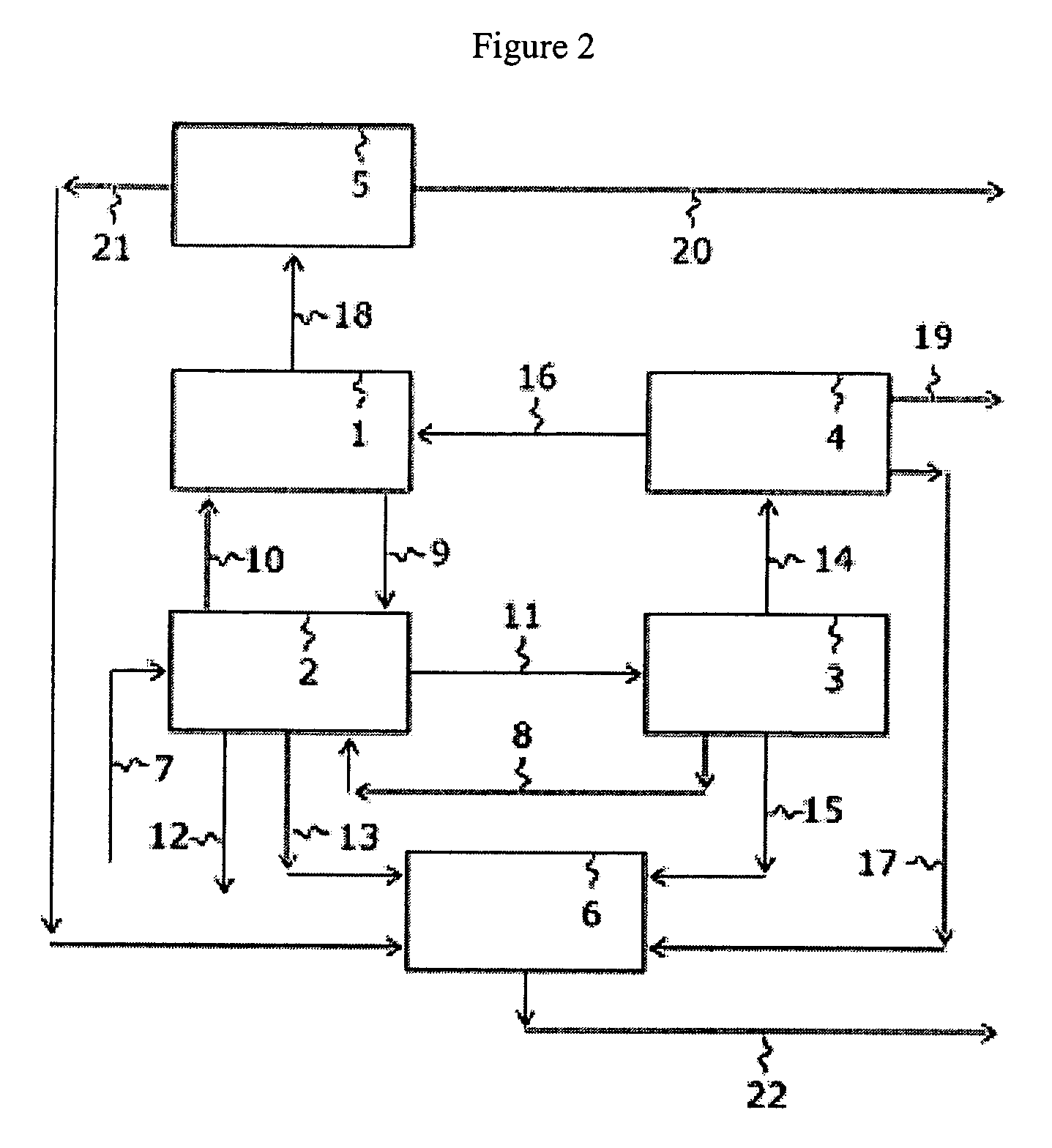
Nitrogen trifluoride production apparatus
InactiveUS6010605AHigh purityReduce impurityElectrolysis componentsNitrogen trifluorideHydrogen fluorideBoron trifluoride
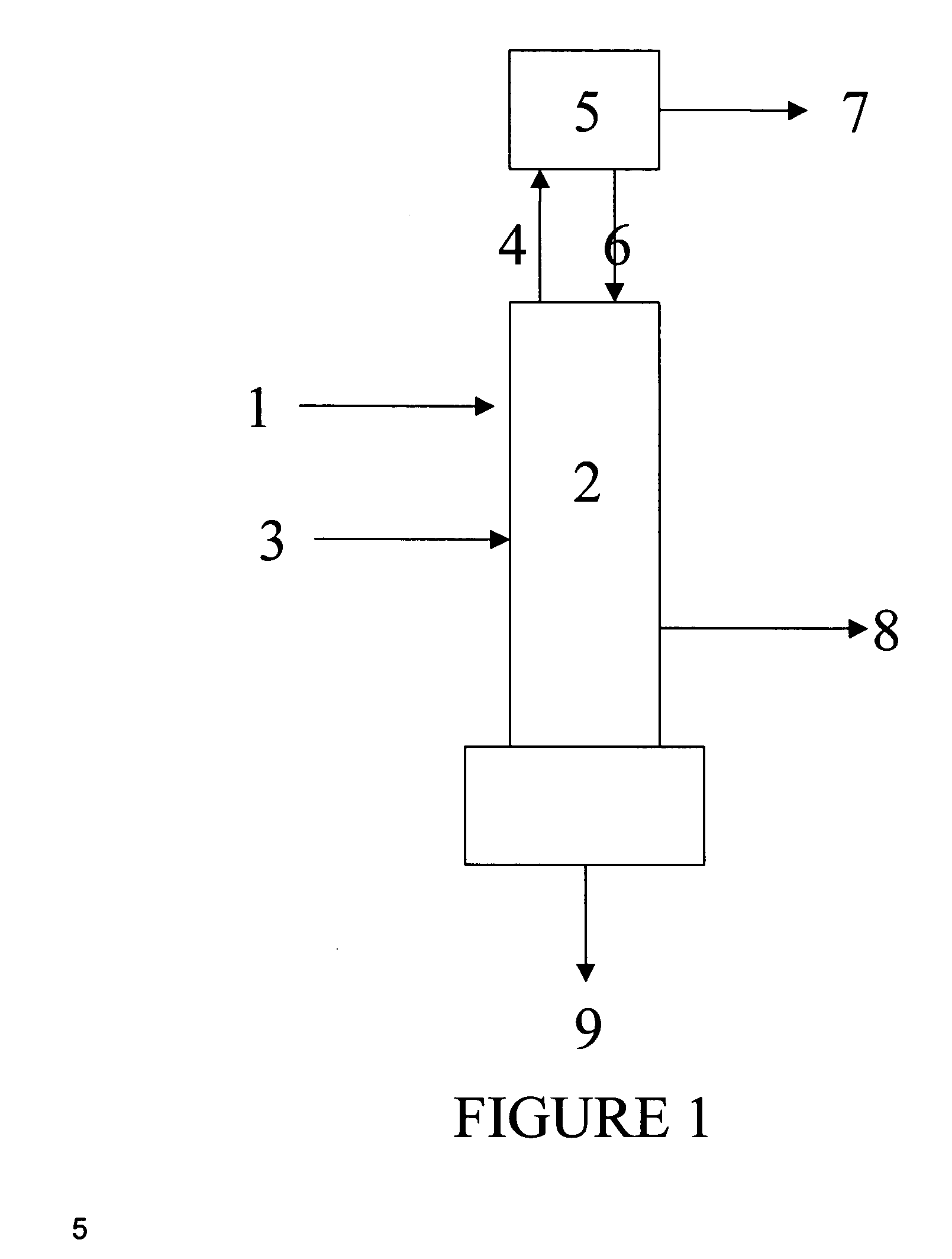
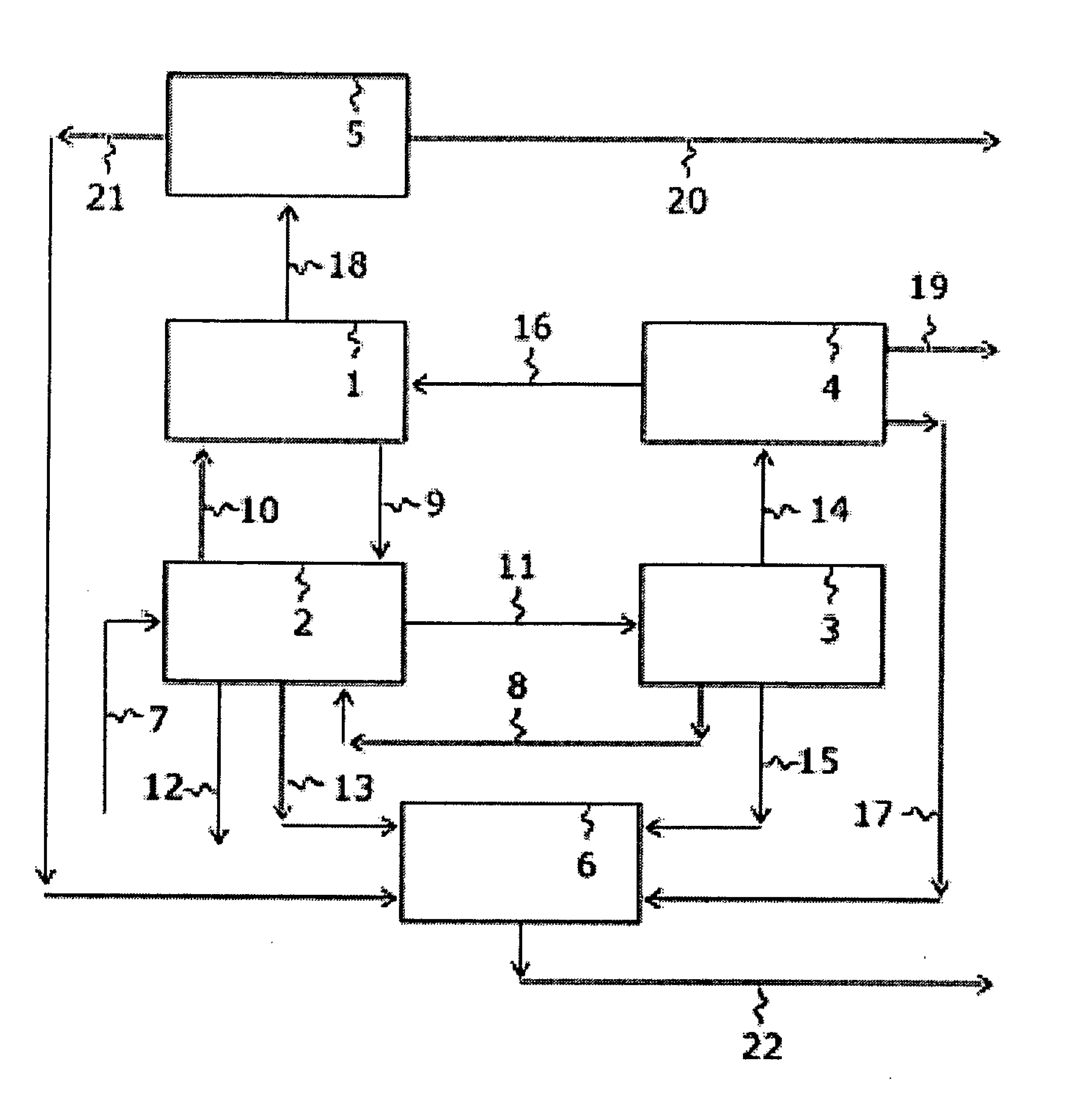
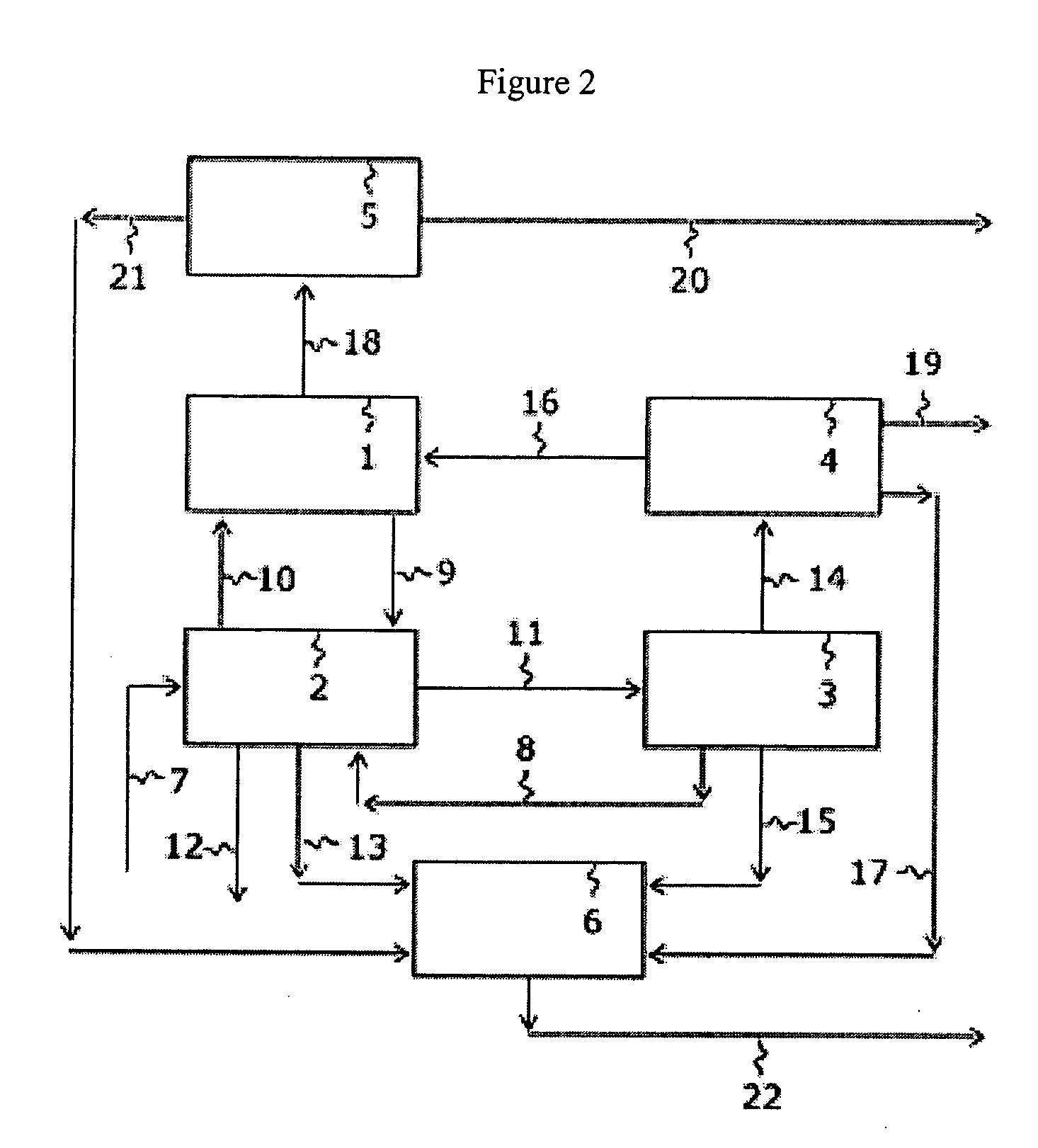
Apparatus is disclosed for the production of nitrogen trifluoride (NF3), starting with an anhydrous molten flux including ammonia (NH3), a metal fluoride (MF), and hydrogen fluoride (HF). The apparatus includes an electrolyzer, an ammonia solubilizer, a hydrogen fluoride solubilizer, a nitrogen trifluoride reactor, two compressors, two pumps, three condensers a gas recycle loop, and, two flux loops of the same component ternary flux, but each loop with different concentration.
Owner:FLORIDA SCI LAB
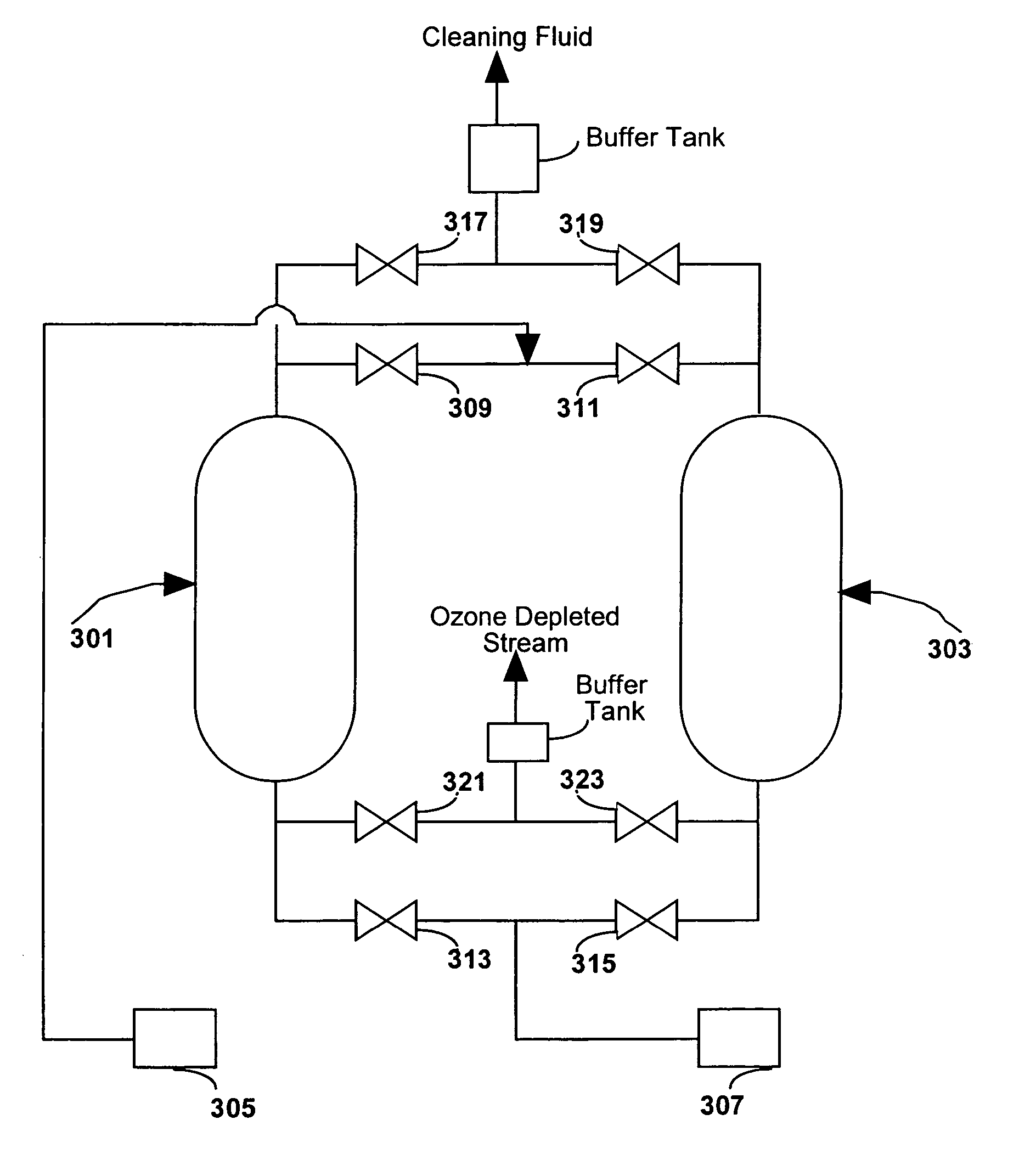
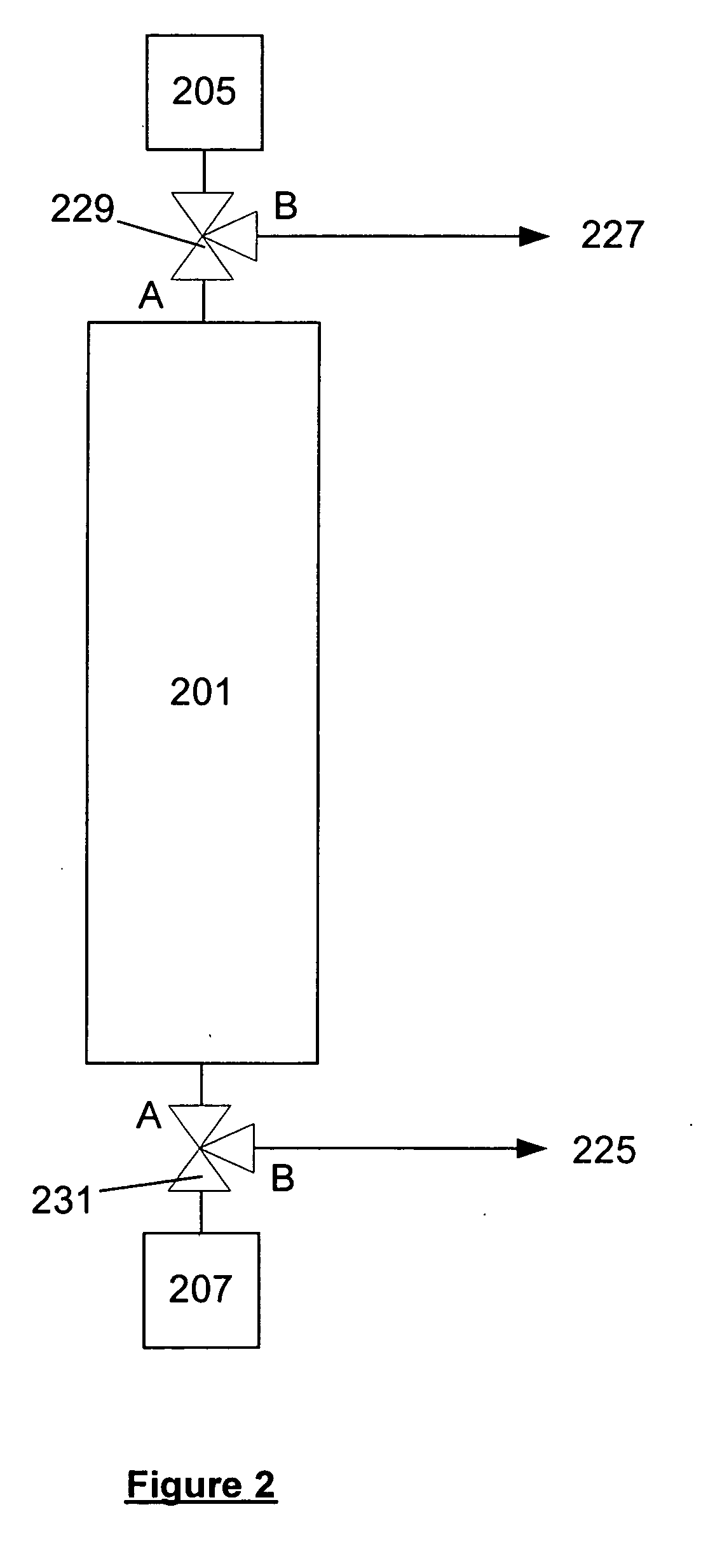
Method of producing a mixture of ozone and high pressure carbon dioxide
Mixtures of an oxidizer and a high pressure fluid are produced by adsorbing an oxidizer in an adsorption bed and then desorbing the oxidizer with a high pressure fluid. The same steps can simultaneously occur in a second adsorbing bed but in reverse order. The oxidizer may be ozone and the high pressure fluid may be high pressure C02 including supercritical C02. Such mixtures can be used for applications such as cleaning semiconductor wafers, food disinfection and water disinfection.
Owner:BOC GRP INC
Fluorine purification
Owner:FLUOROMER
Purification of nitrogen trifluoride
InactiveUS7384618B2Efficient purificationReduce concentrationNitrogen purification/separationNitrogen-metal/silicon/boron binary compoundsMolecular sieveImpurity
A process and system for adsorption purification of NF3 wherein a crude product containing NF3 and impurities such as CF4 is brought into contact with a polyacrylonitrile-based carbon molecular sieve so that at least a portion of one or more impurities are adsorbed by the sieve without a significant adsorption of the NF3.
Owner:HONEYWELL INT INC
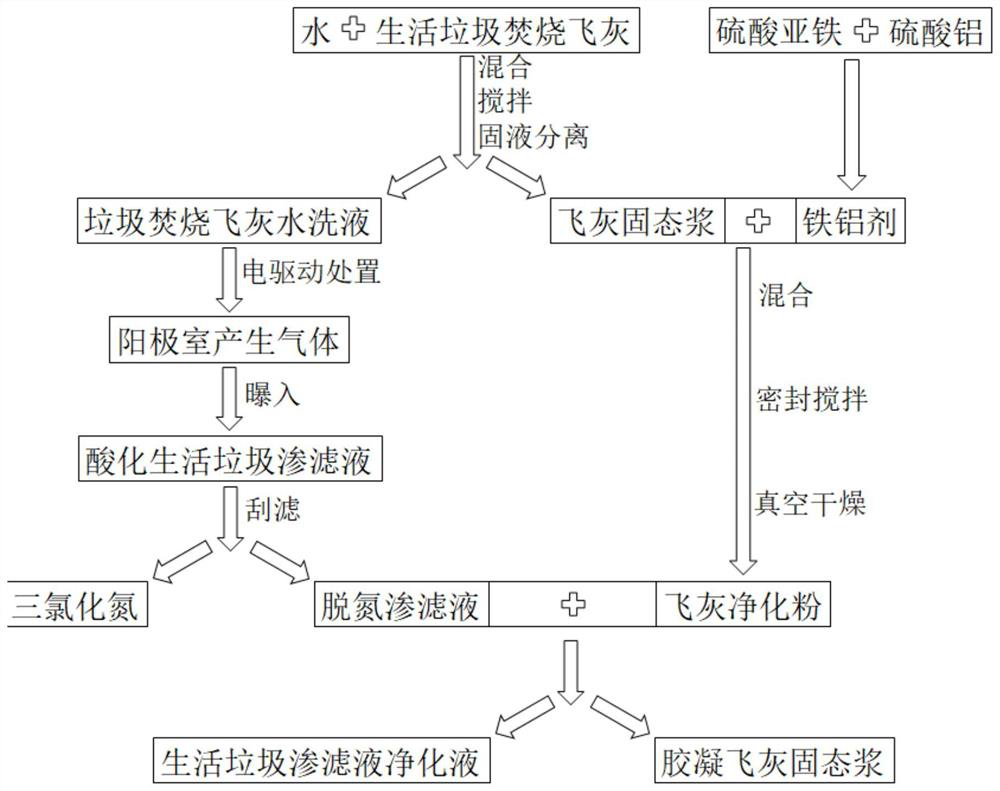
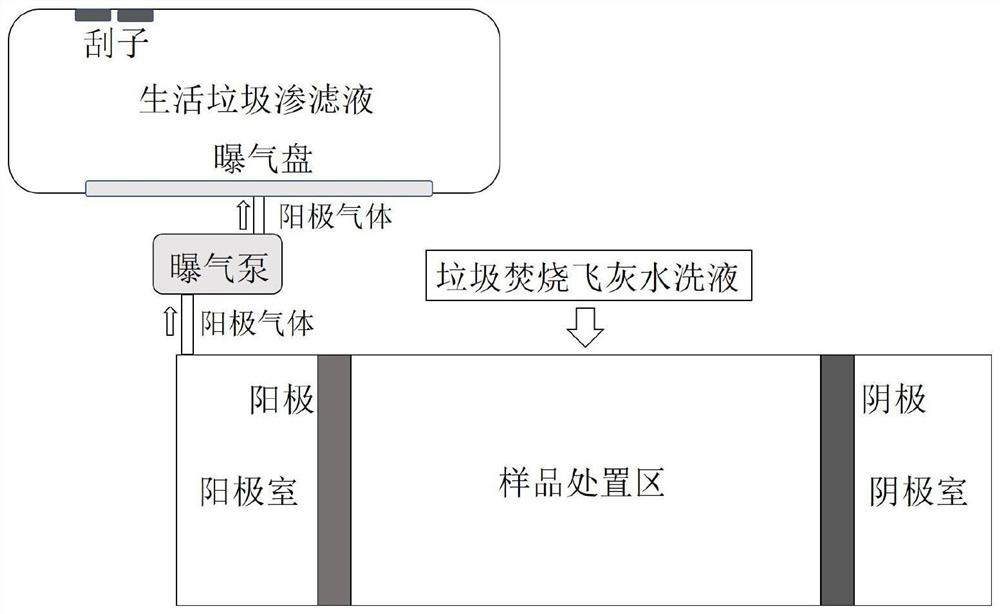
Method for treating household garbage leachate by utilizing household garbage incineration fly ash
InactiveCN111807576AAchieve purificationReduce leaching concentrationWater/sewage treatment by centrifugal separationWater treatment parameter controlWater chlorinationSlurry
The invention discloses a method for treating household garbage leachate by using household garbage incineration fly ash. The method comprises the steps: adding a hydrochloric acid aqueous solution into the household garbage leachate; respectively weighing water and the household garbage incineration fly ash, mixing, stirring and centrifuging for solid-liquid separation to obtain household garbageincineration fly ash water washing liquid and fly ash solid slurry; carrying out electric treatment on the household garbage incineration fly ash water washing liquid, capturing gas generated by an electric anode chamber, directly introducing the gas into the acidified household garbage leachate, and aerating the household garbage leachate; scraping and filtering the household garbage leachate toobtain oily nitrogen trichloride and denitrified leachate; weighing an iron-aluminum agent and the fly ash solid slurry, and carrying out vacuum drying to obtain fly ash purified powder; and weighingthe denitrification leachate and the fly ash purification powder, mixing, stirring, and carrying out solid-liquid separation to obtain the household garbage leachate purification liquid and gelatinized fly ash solid slurry. 86% of nitrogen trichloride can be recycled at most, and 98% of ammonia nitrogen, 97% of COD, 98% of total phosphorus and 98% of mercury in the landfill leachate can be removed.
Owner:浙江中陶环保科技集团有限公司
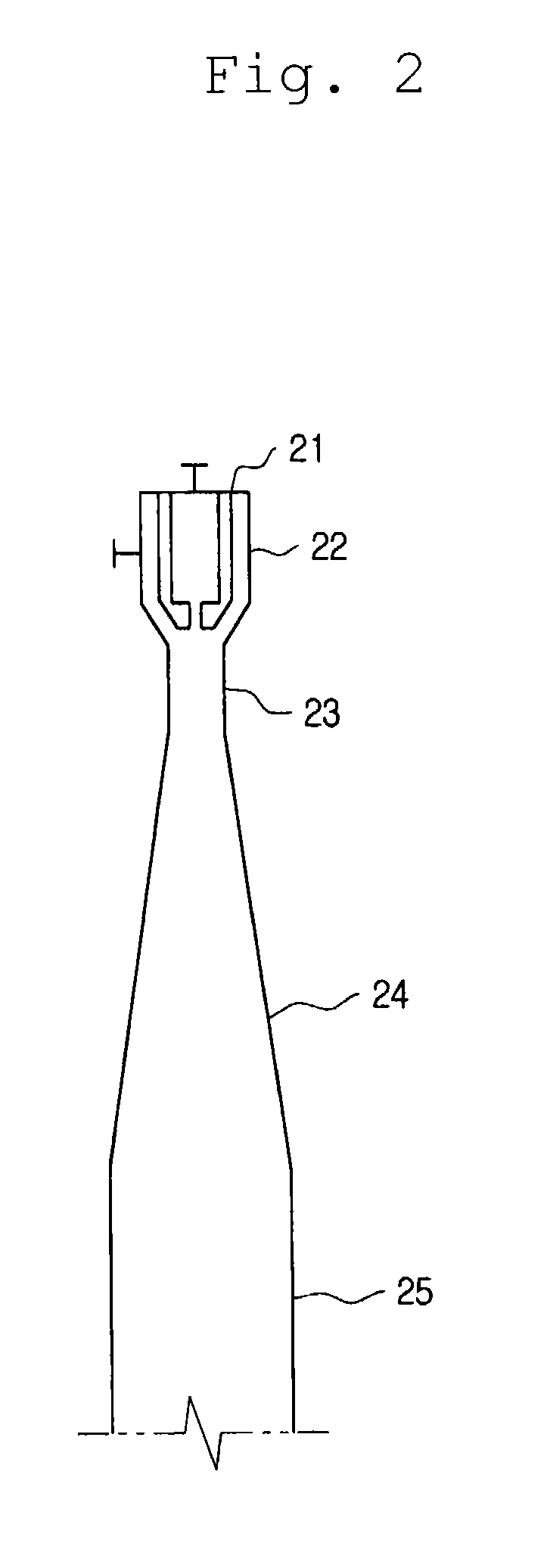
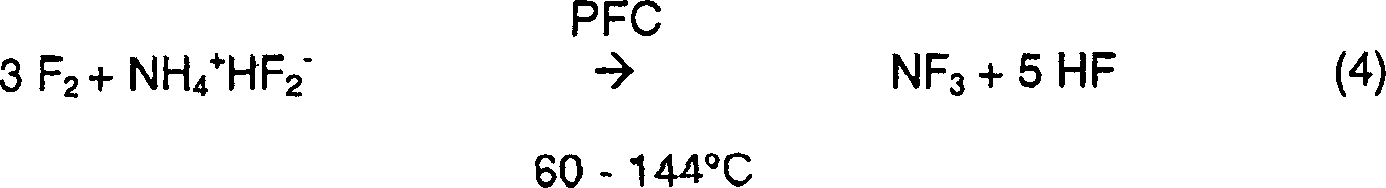
Method for producing nitrogen trifluoride using jet-loop reactors
InactiveUS7083773B2Increasing NF yieldEasy to controlNitrogen-metal/silicon/boron binary compoundsFluorine/hydrogen-fluorideReaction temperatureSide reaction
Nitrogen trifluoride is produced with a high yield by the method comprising forming a fast stream of micro droplets of a fused ammonium fluoride salt by rapidly ejecting the fused ammonium fluoride salt into a reactor through a nozzle while circulating the fused ammonium fluoride salt in the reactor from a lower portion to an upper portion; and contacting micro droplets of the fused ammonium fluoride salt with fluorine gas sucked in the reactor through a suction pipe for fluorine by a negative pressure formed around the nozzle due to an ejection of the fused ammonium fluoride salt, whereby excessive generation and regional accumulation of the heat of reaction are prevented, reducing the reaction temperature by 10˜30° C. compared with those of the existing methods, and a side reaction occurs only to a slight extent according to the lowered reaction temperature.
Owner:KOREA INST OF SCI & TECH
NF3 production reactor
InactiveUS7128885B2Reduce corrosionHigh energy inputCombination devicesFlow mixersBoron trifluorideHigh energy
A process for the production of nitrogen trifluoride by reacting fluorine gas and liquid ammonia acid fluoride in a first reaction zone having a relatively low energy input followed by treatment of the resulting reaction product in a second reaction zone having a relatively high energy input. The resulting crude nitrogen trifluoride product may be further treated with fluorine gas under elevated temperatures to improve yield of the desired product.
Owner:BOC GRP INC
Method and apparatus for removing contaminants from nitrogen trifluoride
InactiveCN103588183AGood removal effectLow impurity contentNitrous oxide captureDispersed particle separationBoron trifluorideImpurity
A highly pure nitrogen trifluoride process fluid having an impurity content of 10 ppm or less can be effectively obtained by using radiation to cause the dissociation of chemical bonds in the impurity to form dissociation products and thereby make the removal of the dissociation products from the process fluid easier than the removal of the impurity from the process fluid.
Owner:AIR PROD & CHEM INC
Process for producing nitrogen trifluoride and use thereof
InactiveUS20030017098A1Improve securityImprove efficiencyNitrogen-metal/silicon/boron binary compoundsHydrogen fluorideGas phaseOrganic chemistry
Owner:SHOWA DENKO KK
Distillation process for reducing the concentration of dinitrogen difluoride and dinitrogen tetrafluoride in nitrogen trifluoride
InactiveUS20050016829A1Reduce concentrationReduce the presence of impuritiesDistillation regulation/controlVacuum distillation separationDistillationBoiling point
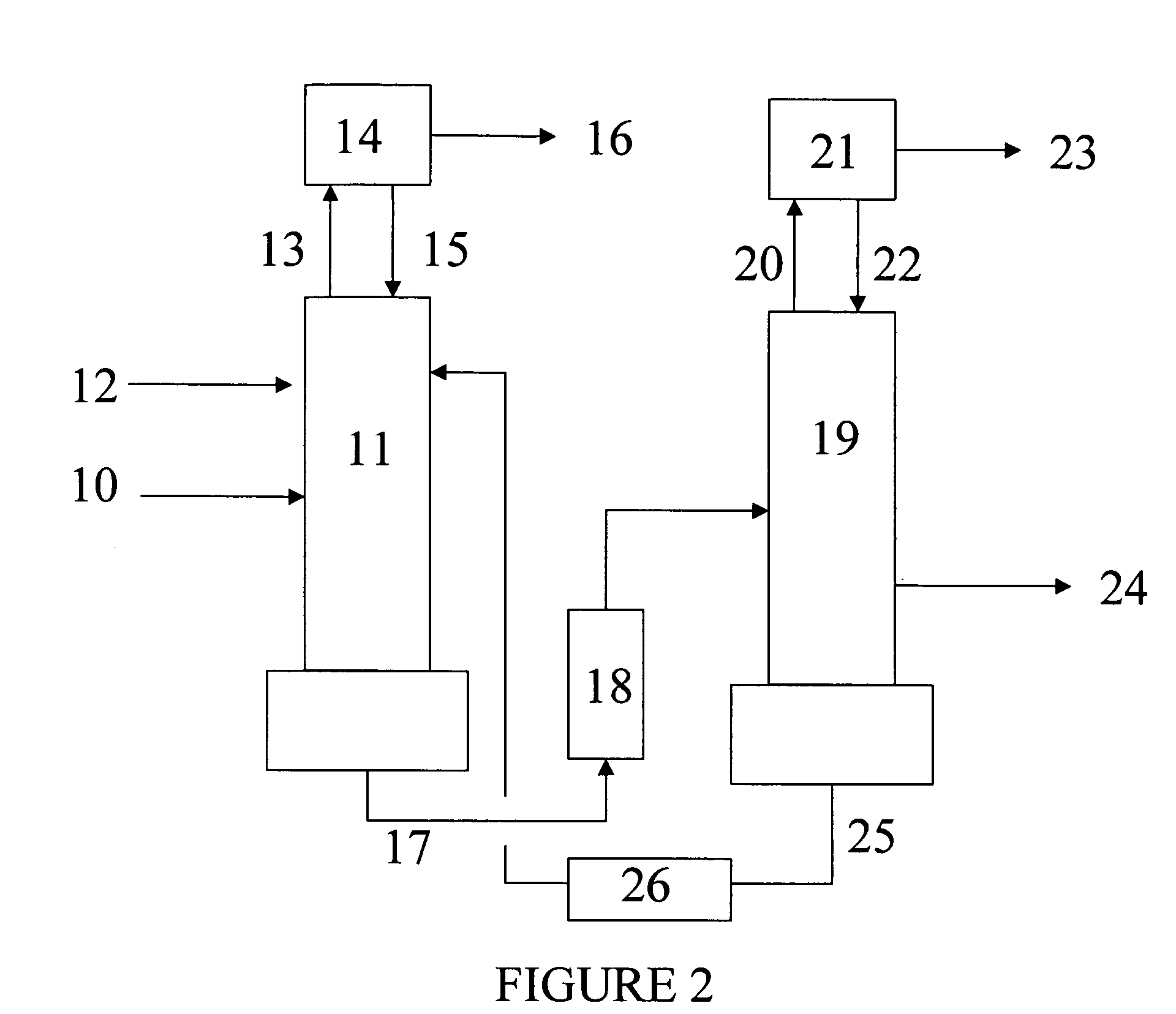
Disclosed is a distillation process for reducing the concentration of impurities dinitrogen difluoride and dinitrogen tetrafluoride in nitrogen trifluoride. The process comprises: (a) distilling a mixture comprising nitrogen trifluoride and dinitrogen difluoride and / or dinitrogen tetrafluoride in a distillation column in the presence of a compound having a higher normal boiling point than nitrogen trifluoride; (b) removing a mixture comprising dinitrogen difluoride and / or dinitrogen tetrafluoride as a sidedraw from the distillation column; (c) removing a mixture comprising the compound having a higher normal boiling point than nitrogen trifluoride from the bottom of the distillation column; and (d) removing a nitrogen trifluoride product having reduced concentration of dinitrogen difluoride and / or dinitrogen tetrafluoride from the top of the distillation column.
Owner:EI DU PONT DE NEMOURS & CO
Purification of nitrogen trifluoride
InactiveUS20060228285A1Efficient purificationReduce concentrationNitrogen purification/separationNitrogen-metal/silicon/boron binary compoundsMolecular sieveImpurity
A process and system for adsorption purification of NF3 wherein a crude product containing NF3 and impurities such as CF4 is brought into contact with a polyacrylonitrile-based carbon molecular sieve so that at least a portion of one or more impurities are adsorbed by the sieve without a significant adsorption of the NF3.
Owner:HONEYWELL INT INC
Purification of nitrogen trifluoride
InactiveCN101189185ANitrogen purification/separationNitrogen trifluorideMolecular sievePhysical chemistry
A process and system for adsorption purification of NF3 wherein a crude product containing NF3 and impurities such as CF4 is brought into contact with a polyacrylonitrile-based carbon molecular sieve so that at least a portion of one or more impurities are adsorbed by the sieve without a significant adsorption of the NF3.
Owner:HONEYWELL INT INC
Method of purifying gaseous nitrogen trifluoride
Owner:ZAKRYTOE AKTSIONERNOE OBSCHESTVO NAUCHNO PROIZVODSTVENNOE OBIEDINENIE PIM INVEST
Method for purifying nitrogen trifluoride
ActiveUS7569122B2Effectively and economically obtainedNitrogen-metal/silicon/boron binary compoundsDistillation separationVaporizationLiquid nitrogen
A highly pure nitrogen trifluoride having a carbon tetrafluoride content of 10 ppm or less can be effectively obtained by boiling crude liquid nitrogen trifluoride having carbon tetrafluoride contaminant under a pressure ranging from 35 to 45 atm, to remove carbon tetrafluoride therefrom through vaporization.
Owner:SK SPECIALTY CO LTD
Process and apparatus for producing fluorinated gaseous compound
InactiveCN101896423ASimple deviceLess risk of failureNitrogen trifluorideInter-halogen compoundsProduct gasReaction zone
An apparatus for producing a fluorinated gaseous compound. The apparatus includes a circulation system which comprises: a reaction zone in which a liquid mixture containing a raw-material liquid is reacted with a raw-material gas; a flow zone in which only the liquid mixture flows; an upper transfer zone in which the liquid mixture which has undergone the reaction is transferred from an upper part of the reaction zone to an upper part of the flow zone; and a lower transfer zone in which the liquid mixture is transferred from a lower part of the flow zone to a lower part of the reaction zone. (A) The raw-material gas is introduced into the lower part of the reaction zone, and (B) at least one fluorinated gaseous compound selected between a first fluorinated gaseous compound which is a reaction product yielded in the reaction zone and a second fluorinated gaseous compound obtained by further fluorinating the first fluorinated gaseous compound is introduced into the lower part of the reaction zone. The liquid mixture is circulated by these operations (A) and (B).
Owner:CENT GLASS CO LTD
Process of Fluorinating Inorganic or Organic Compounds by Direct Fluorination
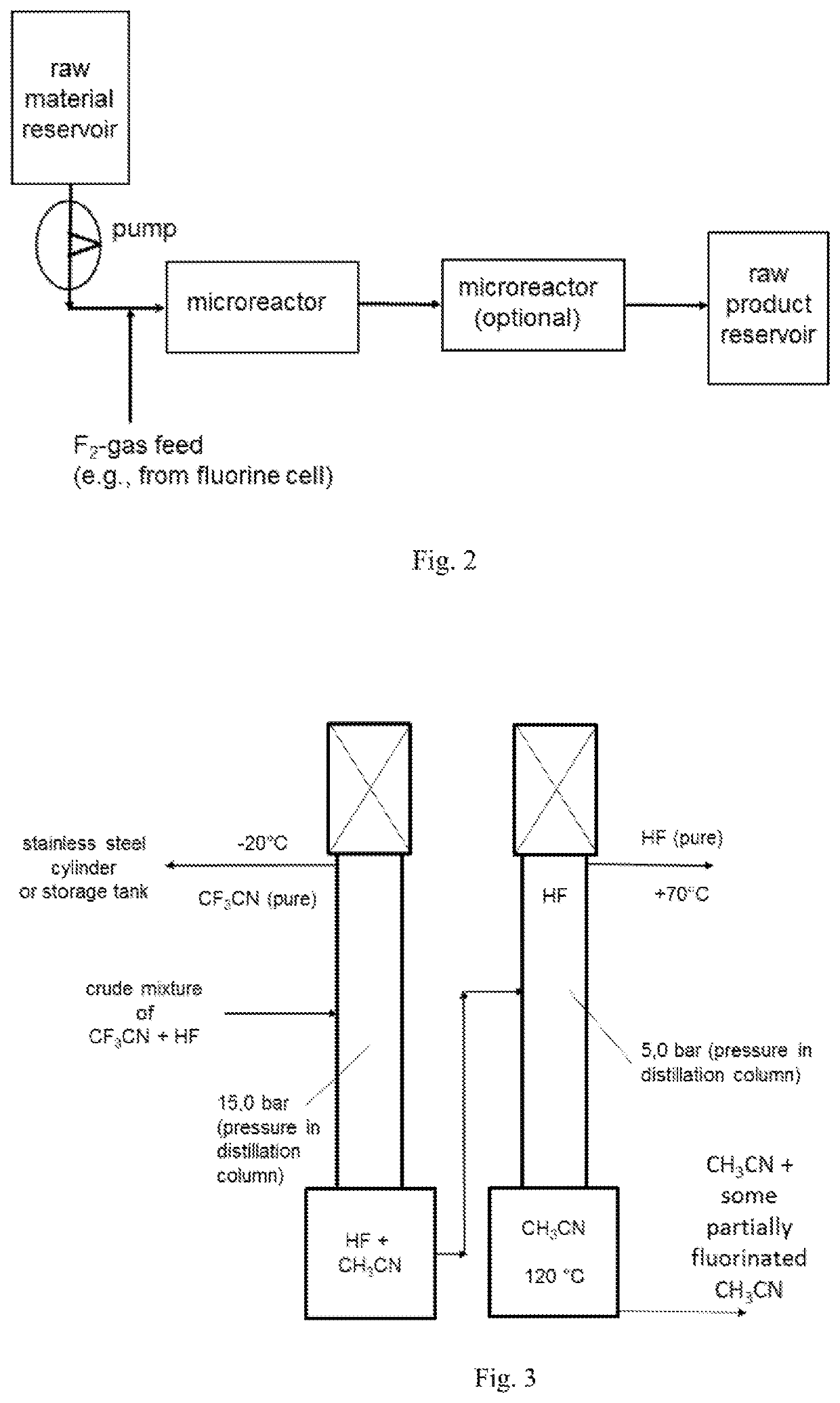
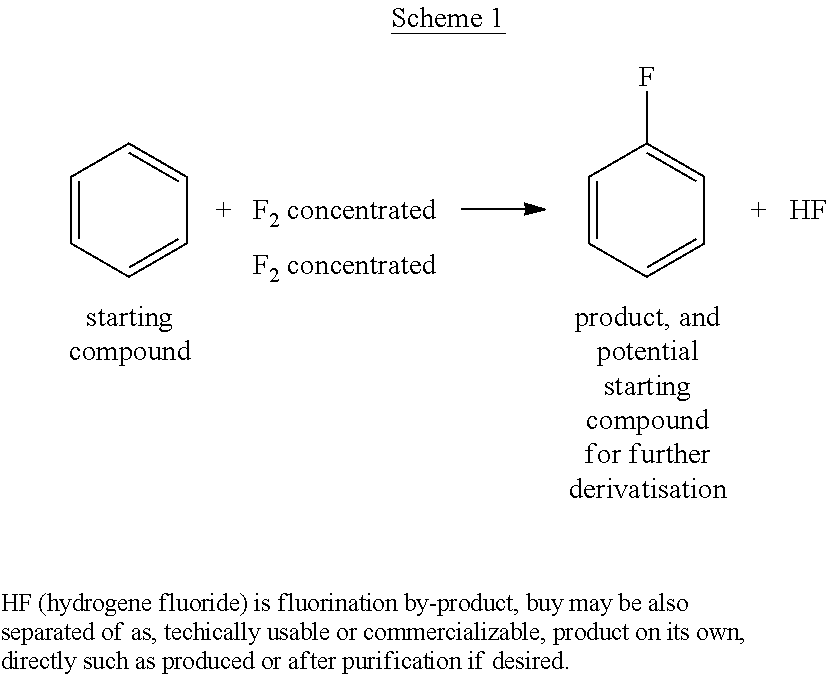
ActiveUS20210053911A1Simplify and reduce numberAvoid a lot of timeCarboxylic acid nitrile preparationOrganic compound preparationBenzeneSimple Organic Compounds
The invention relates to a use of a fluorination gas, and the elemental fluorine (F2) is present in a high concentration, for example, in a concentration of elemental fluorine (F2), especially of equal to much higher than 15 or even 20% by volume, and to a process for the manufacture of a fluorinated compound by direct fluorination employing a fluorination gas, wherein the elemental fluorine (F2) is present in a high concentration. The process of the invention is directed to the manufacture of a fluorinated compound, for the exception of fluorinated benzene, by direct fluorination. Especially the invention is of interest in the preparation of fluorinated organic compounds, final products and as well intermediates, for usage in agro-, pharma-, electronics-, catalyst, solvent and other functional chemical applications. The fluorination process of the invention may be performed batch-wise or in a continuous manner.
Owner:FUJIAN YONGJING TECH CO LTD
Process for refining nitrogen trifluoride gas using alkali earth metal exchanged zeolite
Disclosed herein are a process for the refinement of nitrogen trifluoride gas and an adsorbent used therein. A nitrogen trifluoride (NF3) gas including carbon tetrafluoride (CF4) as an impurity is permeated into a bed of the zeolite 3A, 4A or 5A which is ion exchanged with alkali earth metal and is thermally treated at 150 to 600° C. for 0.5 to 100 hours so as to selectively adsorb nitrogen trifluoride onto the bed, followed by the desorption of the nitrogen trifluoride therefrom.
Owner:HYOSUNG CHEM CORP
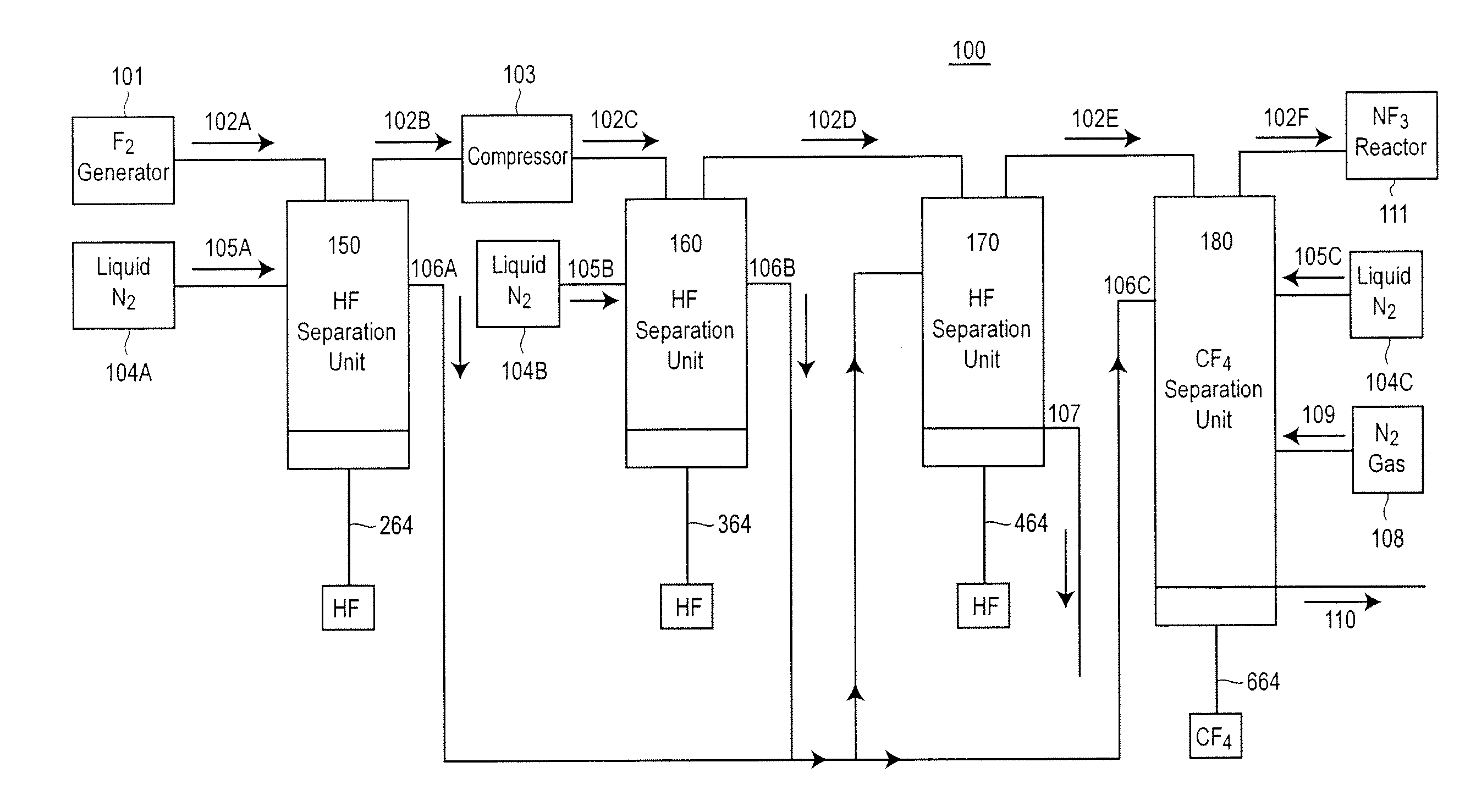
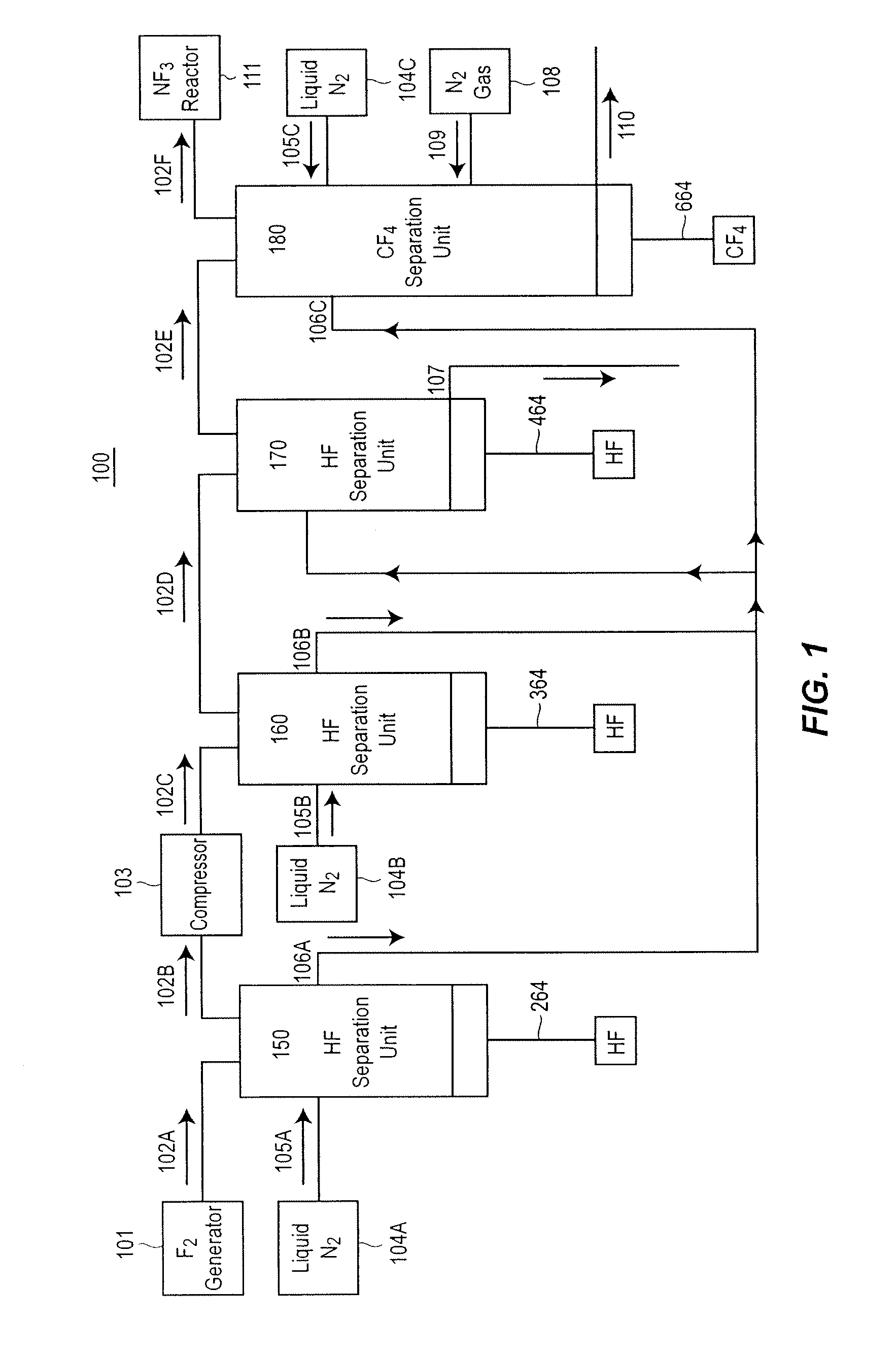
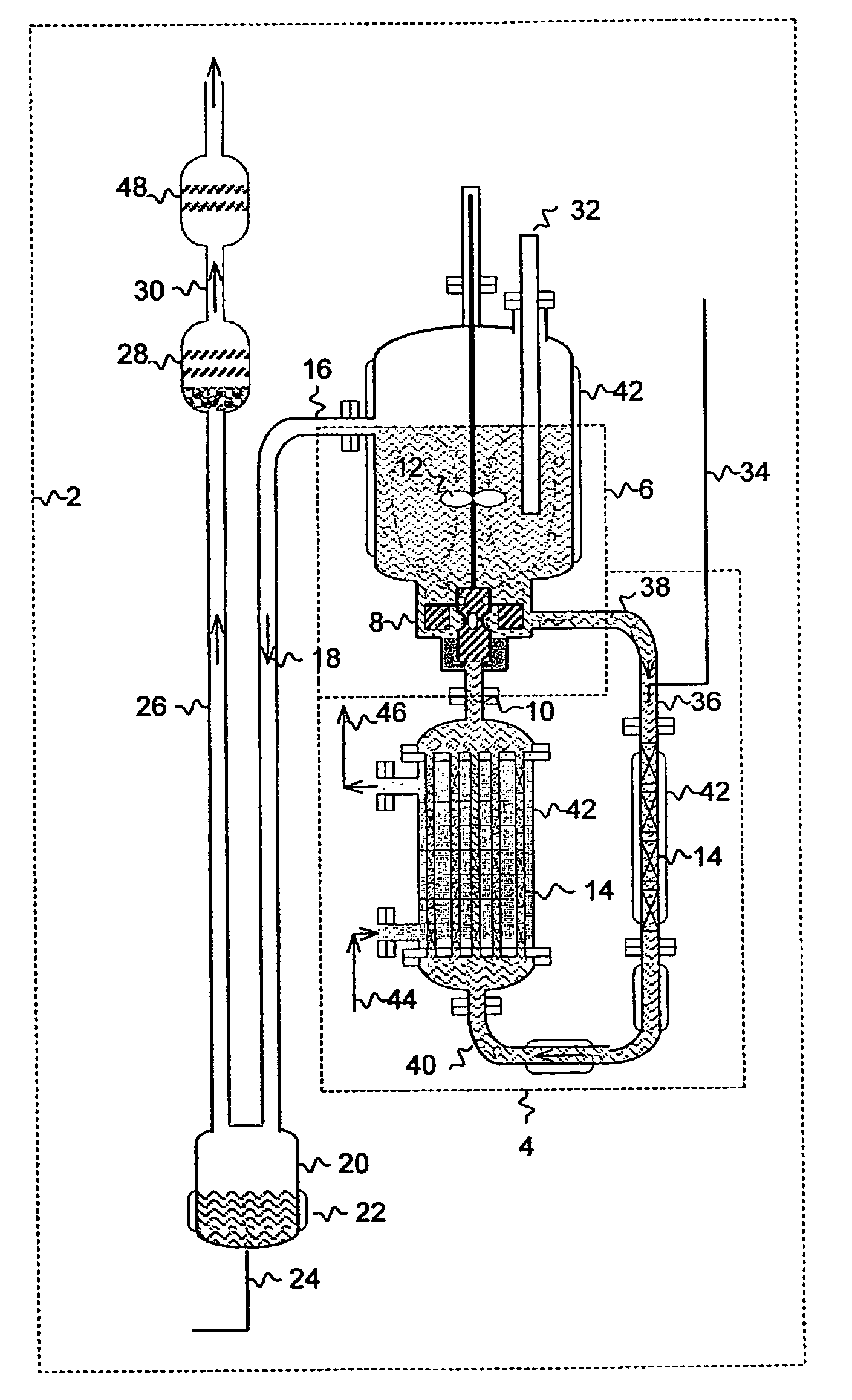
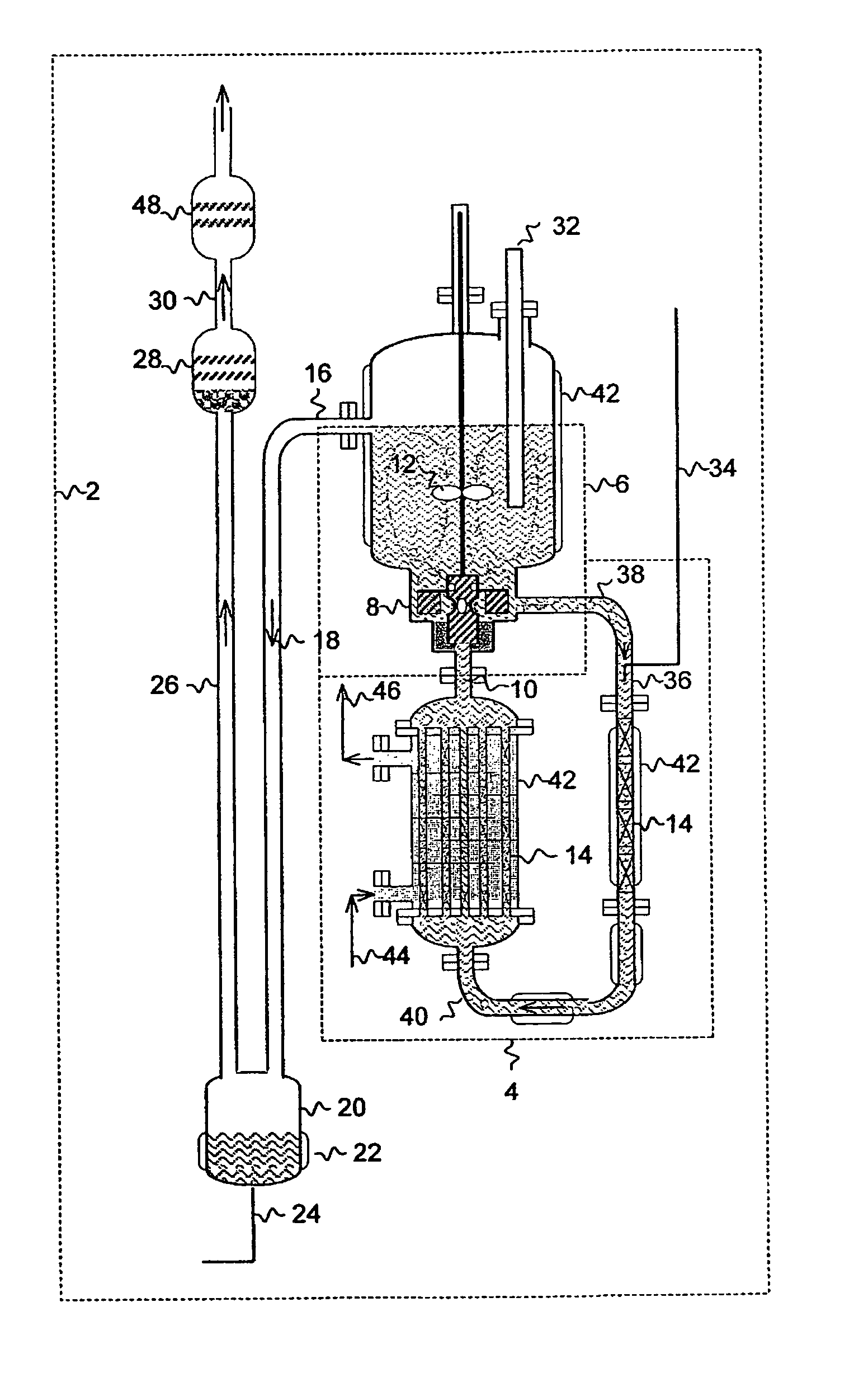
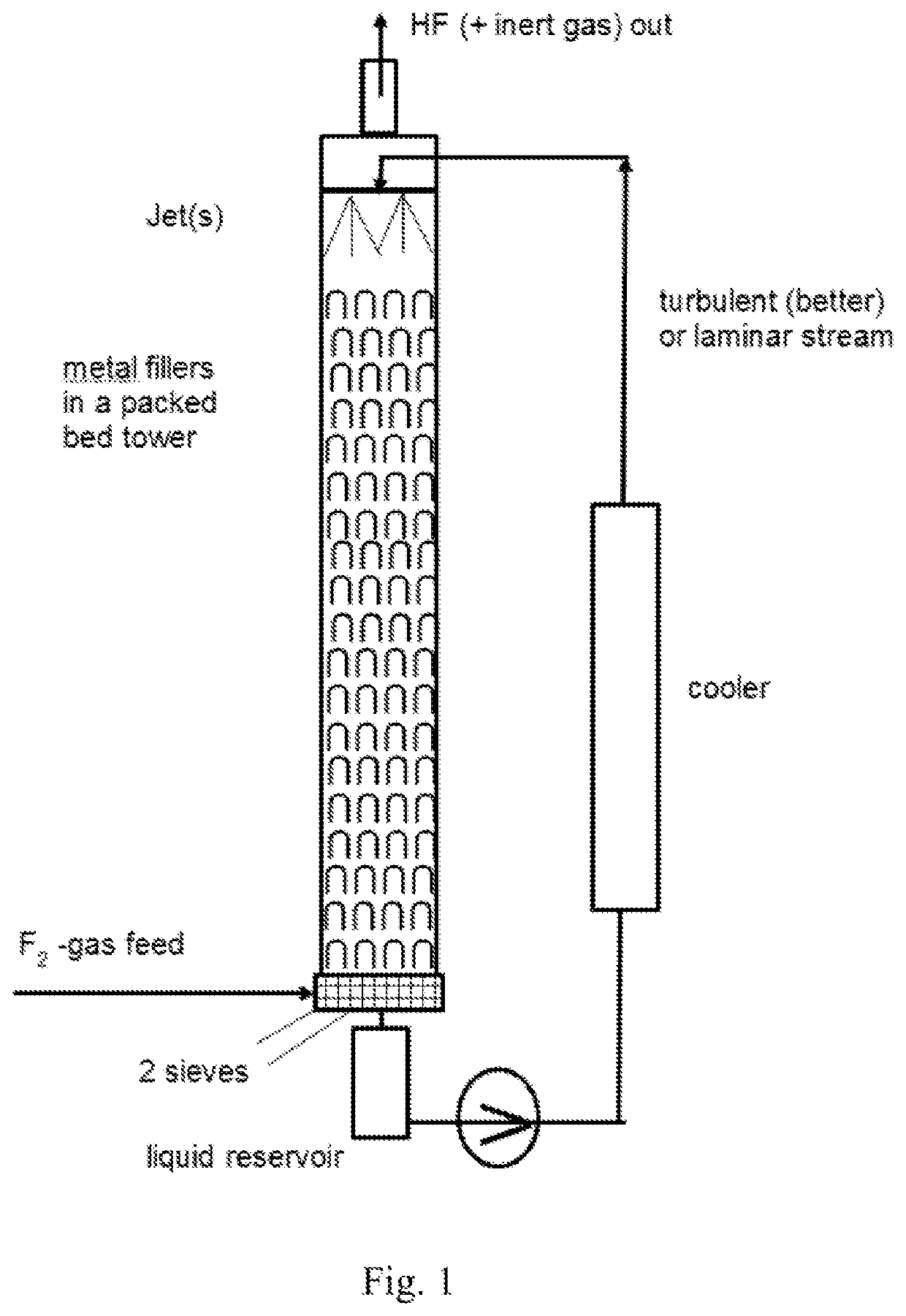
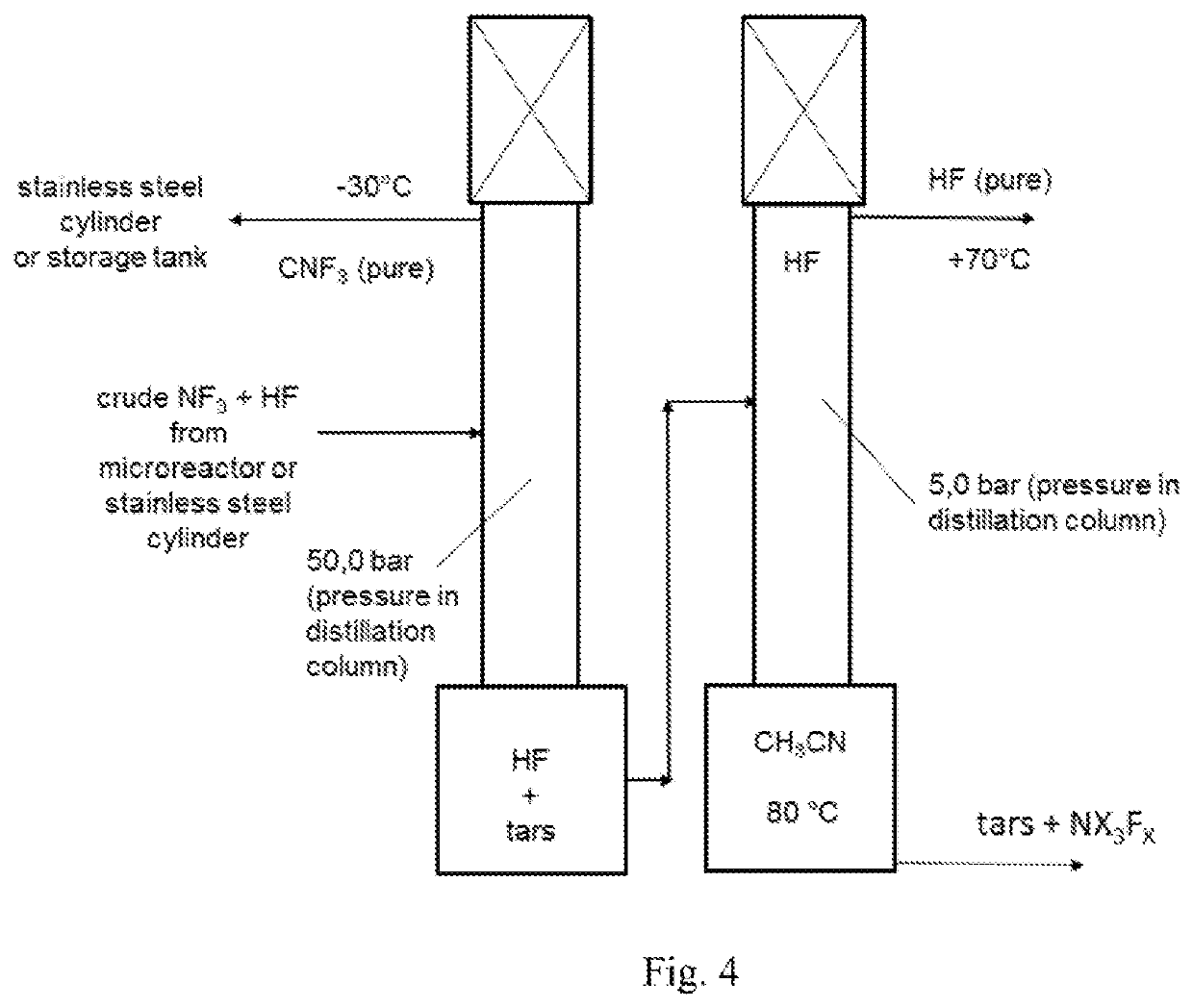
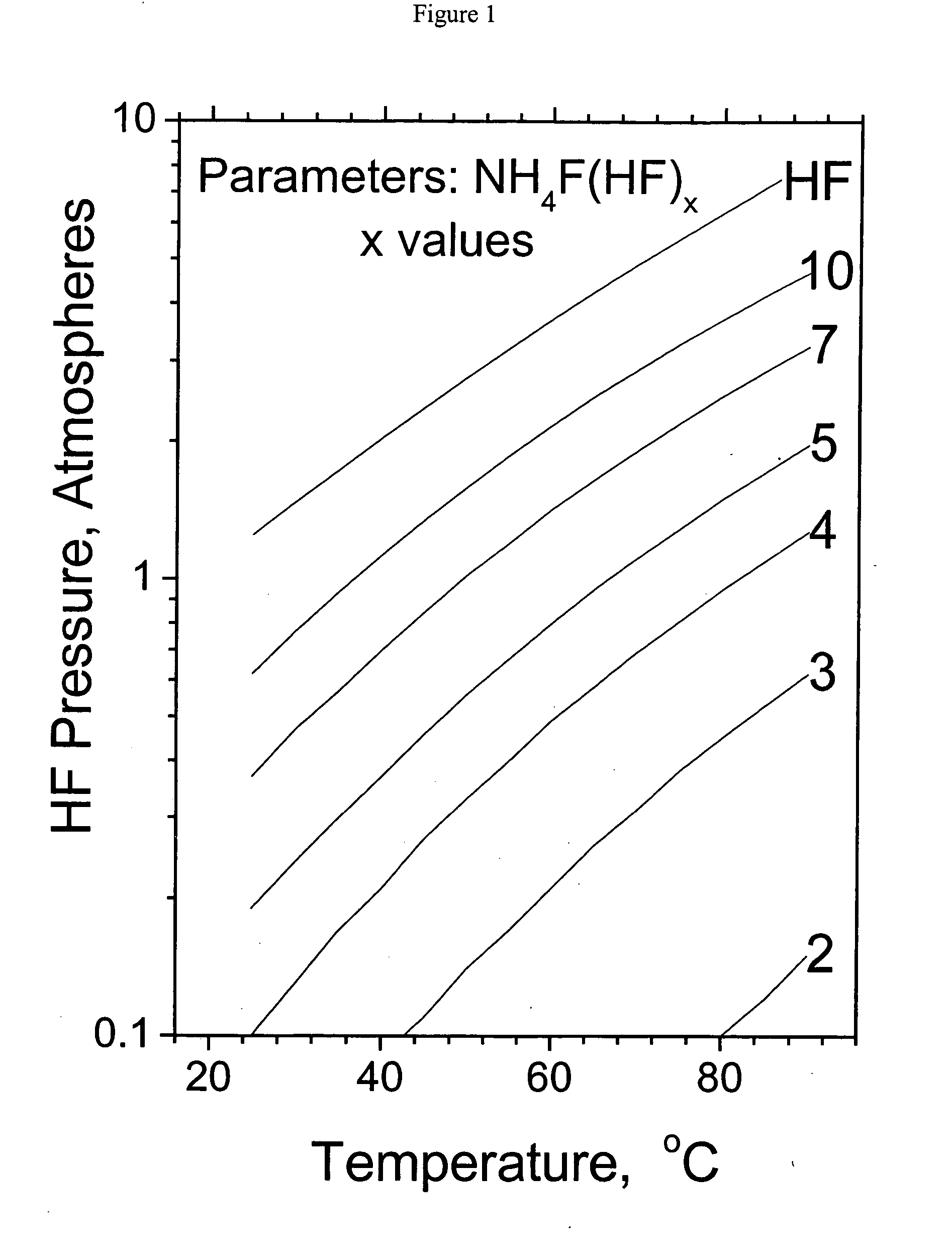
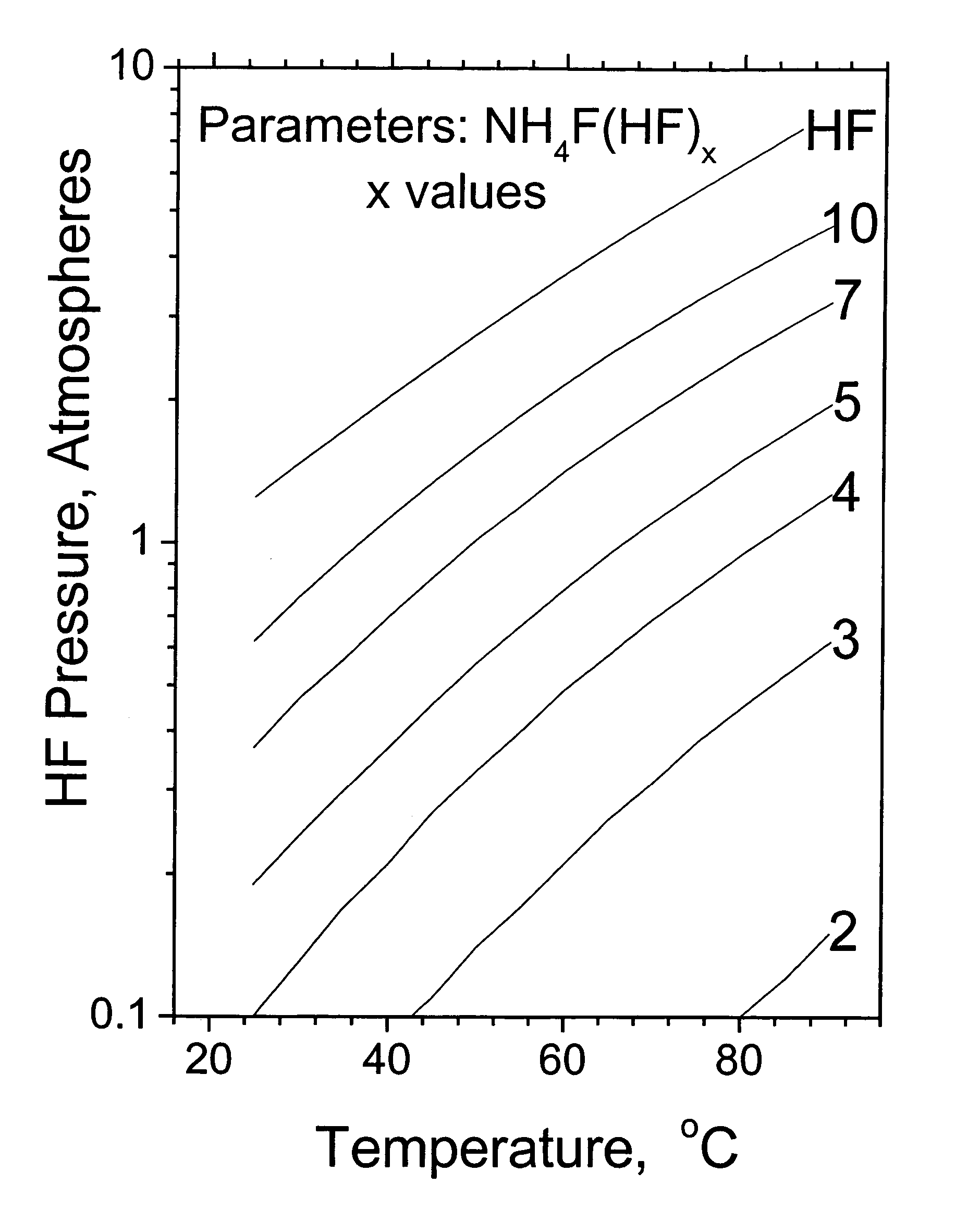
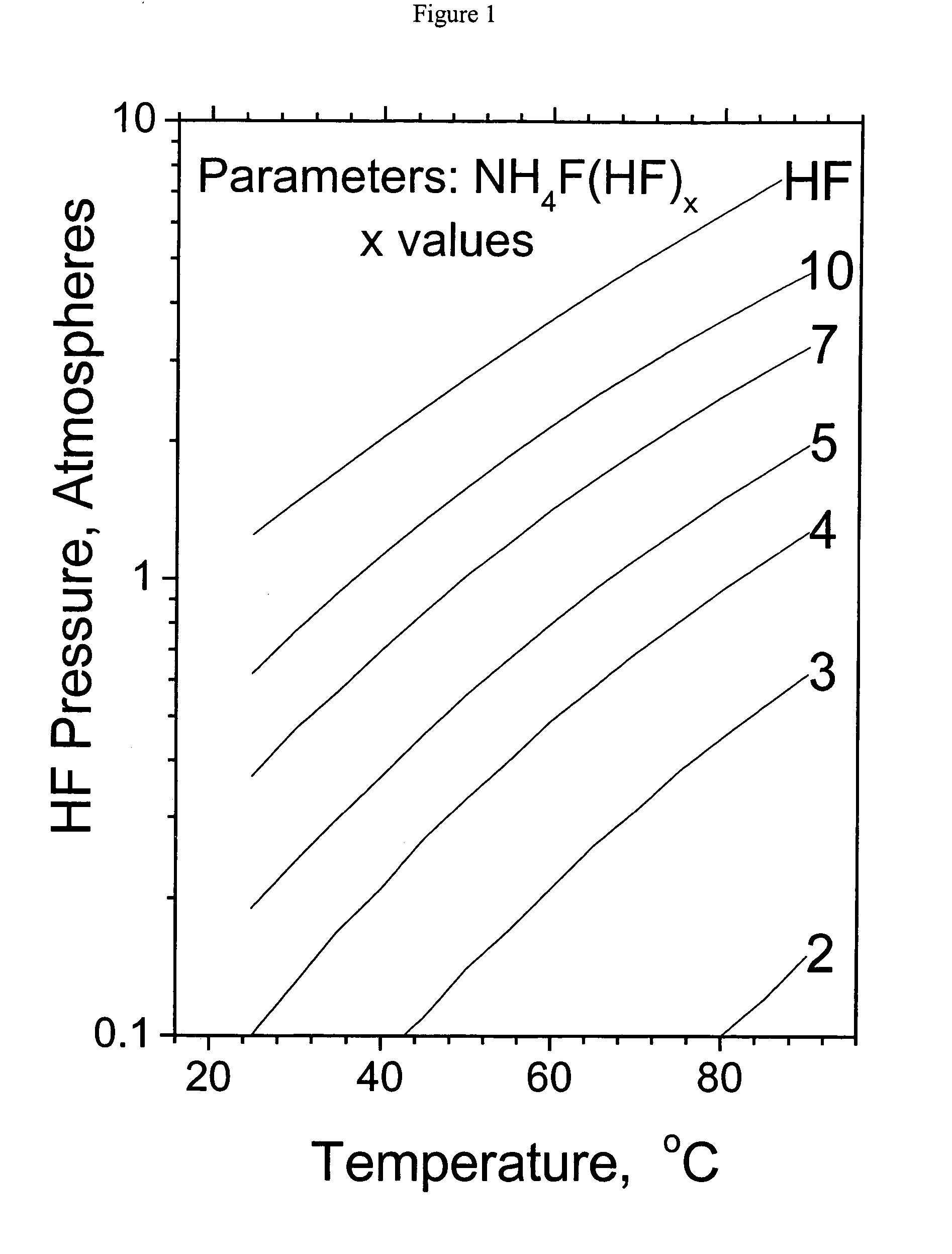
Method and apparatus for local fluorine and nitrogen trifluoride production
InactiveUS20050224093A1Safe deliveryFluoride preparationNitrogen-metal/silicon/boron binary compoundsProcess engineeringSemiconductor
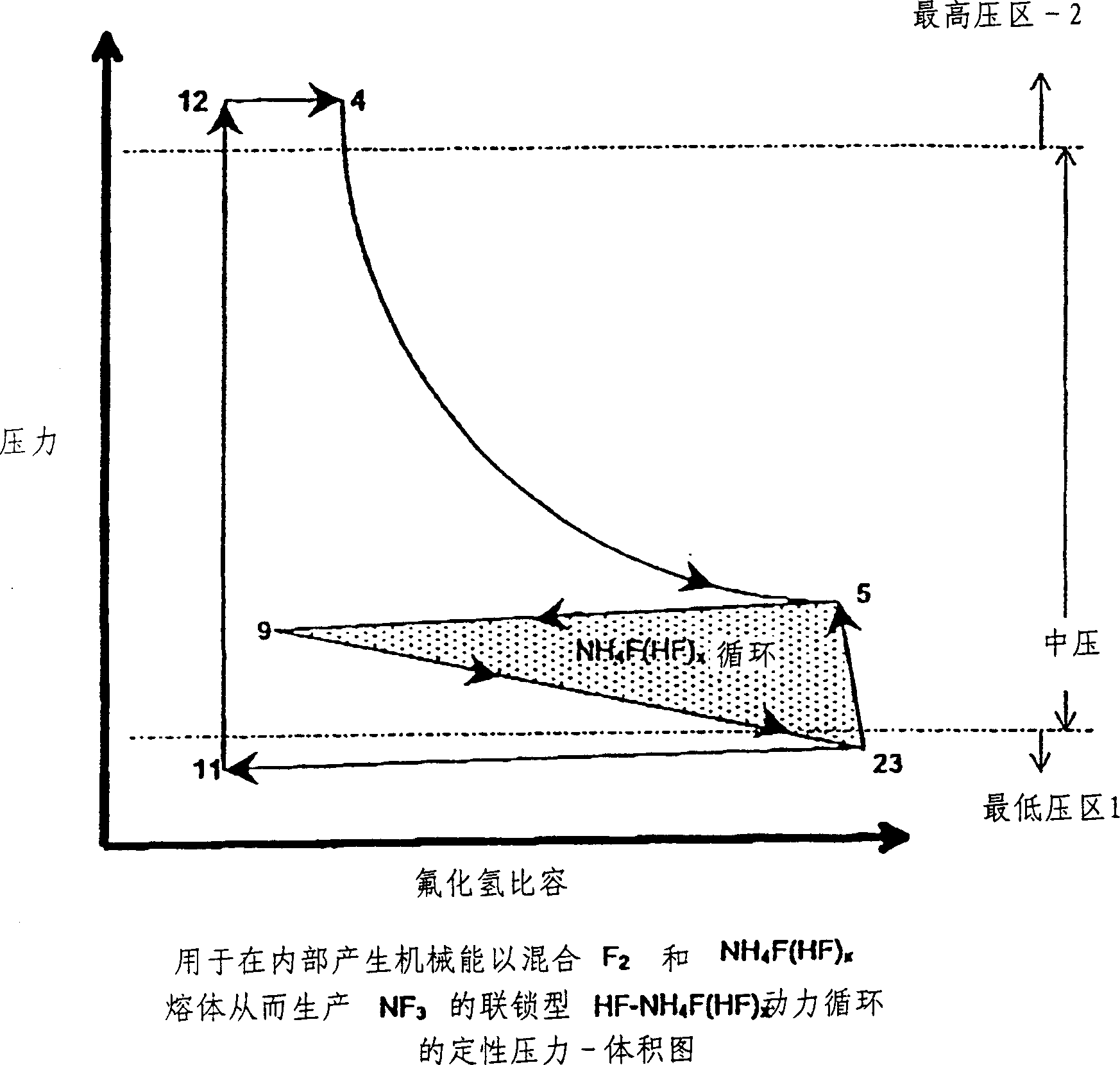
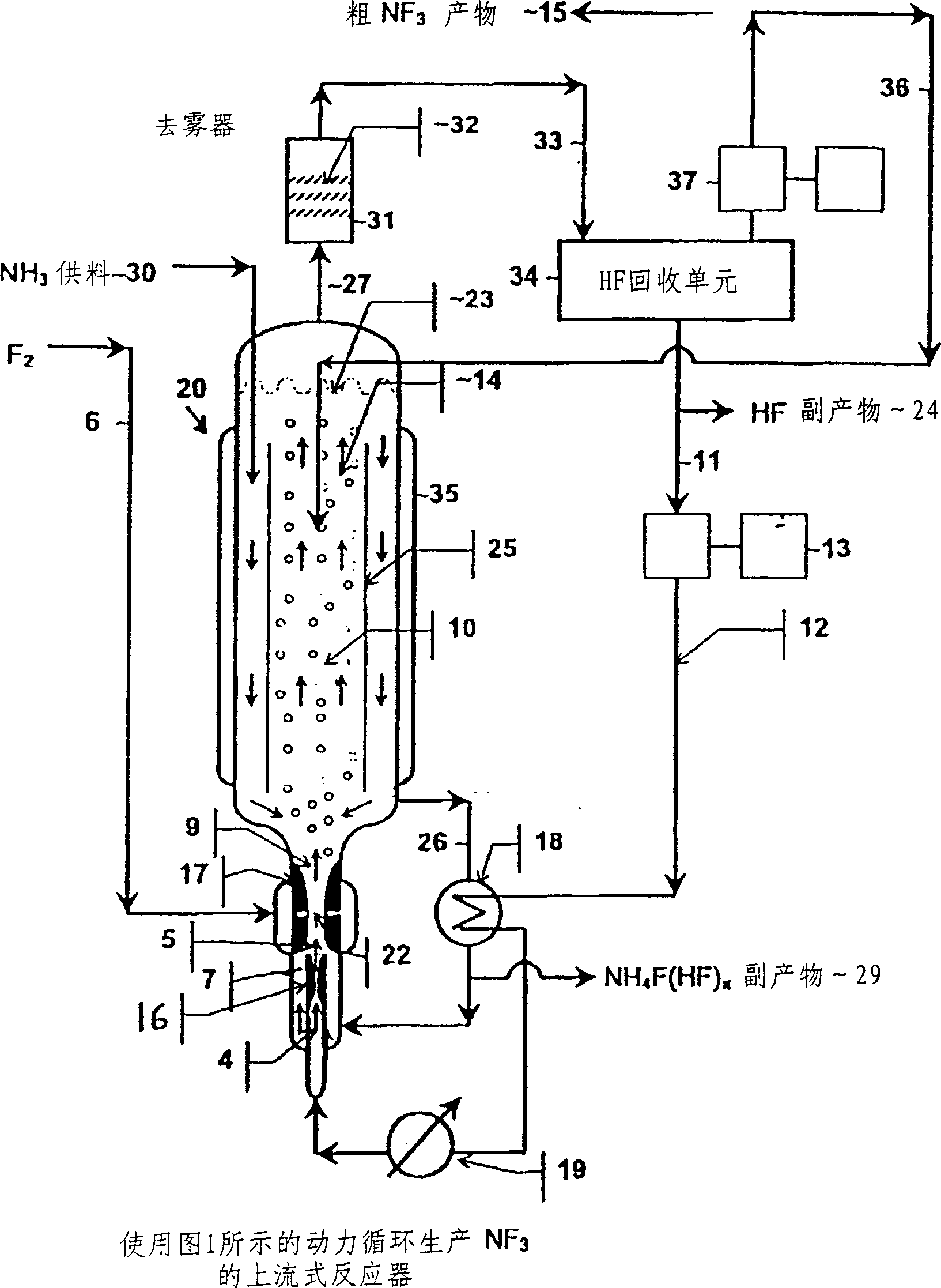
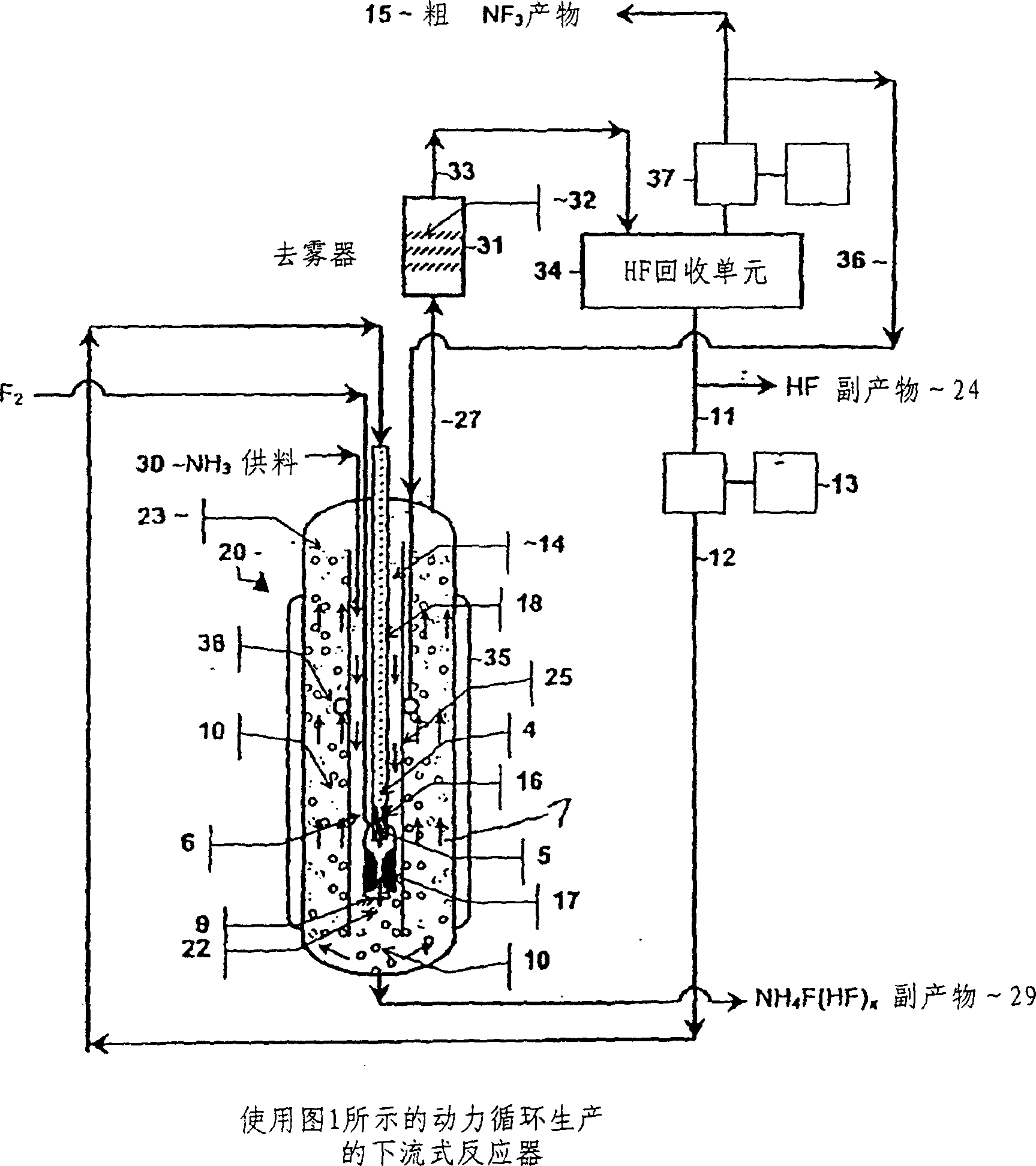
The present invention relates to a process for production, shipment, and treatment of a NH4F(HF)x feedstock for local production of fluorine and NF3 for semiconductor chamber cleaning without the need for storage of large quantities of dangerous feeds and intermediate products.
Owner:BOC GRP INC
Method and apparatus for producing nitrogen trifluoride
InactiveCN1449992ALiquid-gas reaction as foam/aerosol/bubblesFlow mixersHydrogen fluorideMechanical energy
Owner:BOC GRP INC
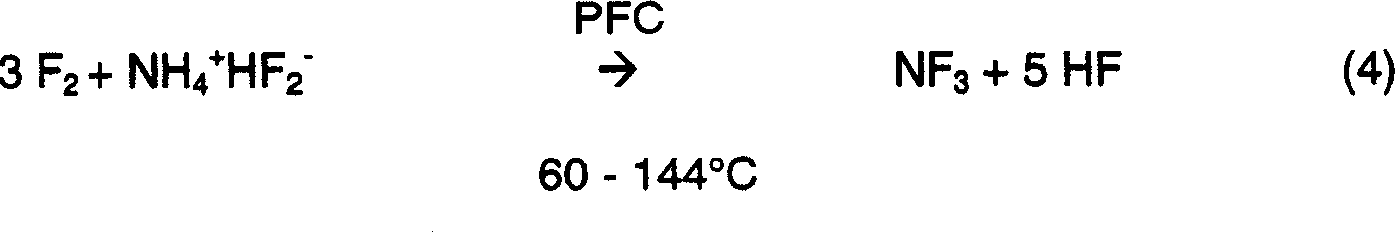
Method for nitrogen trifluoride production
A method for the production of nitrogen trifluoride from a fluorine reactant and an ammonium ion source that is dispersed within a liquid phase reaction mixture containing one or more perfluorocarbon fluids is disclosed herein. In one embodiment, the fluorine reactant is introduced to the reaction mixture at a temperature that ranges from 90 DEG C to 120 DEG C. In this embodiment, the percentage yield of nitrogen trifluoride may be about 80% or greater.
Owner:AIR PROD & CHEM INC
Process for refining nitrogen trifluoride gas using alkali earth metal exchanged zeolite
Owner:HYOSUNG CHEM CORP
Process for refining nitrogen trifluoride gas using alkali earth metal exchanged and impregnated zeolite
Owner:HYOSUNG CHEM CORP
Process for synthesis of halogenated nitrogen
A process for synthesizing a halogenated nitrogen represented by a formula of NFxL3-x, where L represents a halogen other than fluorine, and 1≦x≦3 is provided. This process includes the step of (a) reacting an ammonium complex compound that is one selected from the group consisting of NH4F.nHF, (NH4)yMFz.mHF and a mixture of these and that is in liquid form, with an interhalogen compound or a mixture of an interhalogen compound and F2 gas, wherein 1<n, 1≦y≦4, 2≦z≦8, 0.1≦m, and M represents one selected from the group consisting of elements of group 1 to group 16 of periodic table and a mixed element of these elements.
Owner:CENT GLASS CO LTD
Process for producing nitrogen trifluoride
InactiveUS7018598B2Good safety and efficiency and profitabilityHigh yieldNitrogen-metal/silicon/boron binary compoundsHydrogen fluorideGas phaseOrganic chemistry
Owner:RESONAC HOLDINGS CORPORATION
Method and apparatus for local fluorine and nitrogen trifluoride production
InactiveUS7395823B2Safe deliveryFluoride preparationUsing liquid separation agentProcess engineeringSemiconductor
Owner:BOC GRP INC
Method For Purifying Nitrogen Trifluoride
ActiveUS20090142248A1Effectively and economically obtainedNitrogen-metal/silicon/boron binary compoundsDistillation separationVaporizationLiquid nitrogen
A highly pure nitrogen trifluoride having a carbon tetrafluoride content of 10 ppm or less can be effectively obtained by boiling crude liquid nitrogen trifluoride having carbon tetrafluoride contaminant under a pressure ranging from 35 to 45 atm, to remove carbon tetrafluoride therefrom through vaporization.
Owner:SK SPECIALTY CO LTD
Method for nitrogen trifluoride production
A method for the production of nitrogen trifluoride from a fluorine reactant and an ammonium ion source that is dispersed within a liquid phase reaction mixture containing one or more perfluorocarbon fluids is disclosed herein. In one embodiment, the fluorine reactant is introduced to the reaction mixture at a temperature that ranges from 90 DEG C to 120 DEG C. In this embodiment, the percentage yield of nitrogen trifluoride may be about 80% or greater.
Owner:AIR PROD & CHEM INC
Electrode and production method therefor, and production method for regenerated electrode
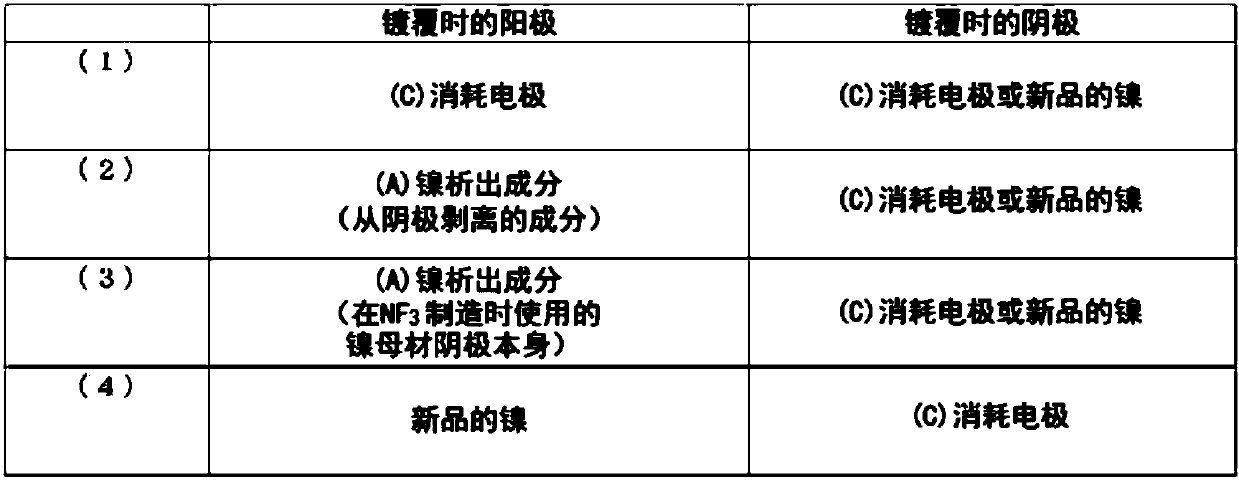
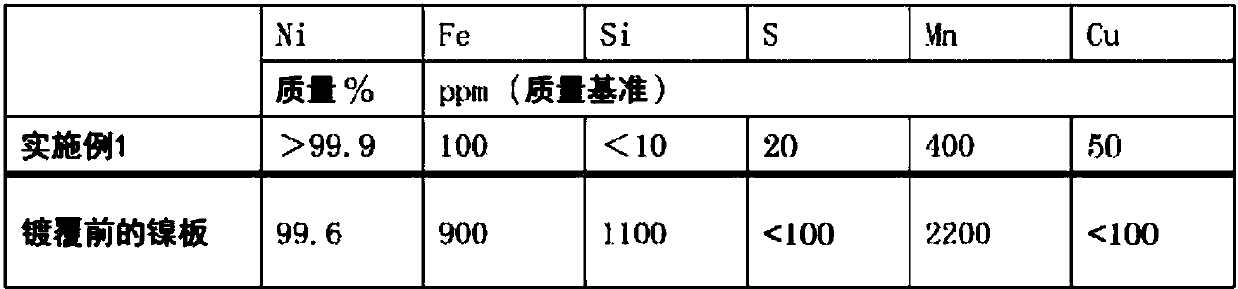
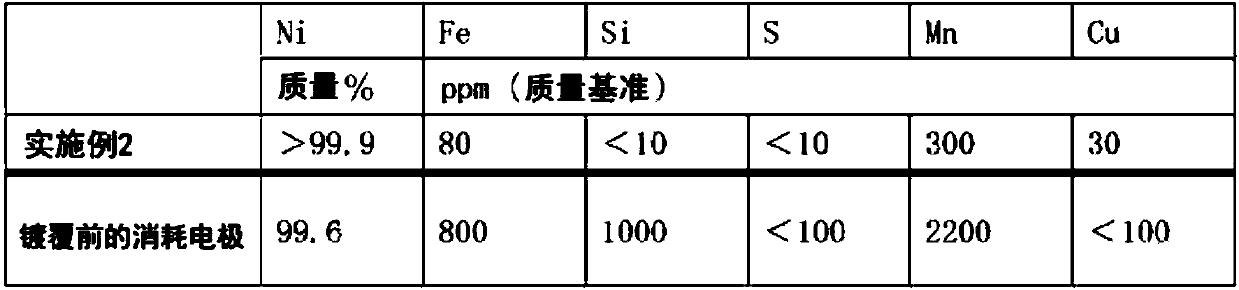
An electrode for electrolytic fluorination according to the present invention contains nickel as a base material, and has a fluorine content of less than 1,000 ppm. The electrode preferably has, in atleast a surface portion thereof, a nickel content of 99 mass% or more, an iron content of 400 ppm or less, a copper content of 250 ppm or less, and a manganese content of 1000 ppm or less. The production method for the electrode according to the present invention comprises disposing a nickel base material electrode as a cathode in a nickel plating bath, and applying nickel plating to the nickel base material electrode by an electrolytic nickel plating method, wherein (1) a nickel component which has precipitated in a molten salt or which has deposited on a cathode in a nitrogen trifluoride production step performed by molten salt electrolysis using a nickel base material anode, or said nickel base material anode, is used an anode, or (2) said nickel base material anode is used as a cathode.
Owner:KANTO DENKA IND CO LTD
Method of storing nitrogen trifluoride
InactiveCN100460745CHigh purityAvoid pollutionGas coolection apparatusPressure vesselsDeep drawingChromium
Owner:FOOSUNG CO LTD
Process for Preparing Fluorobenzene by Direct Fluorination
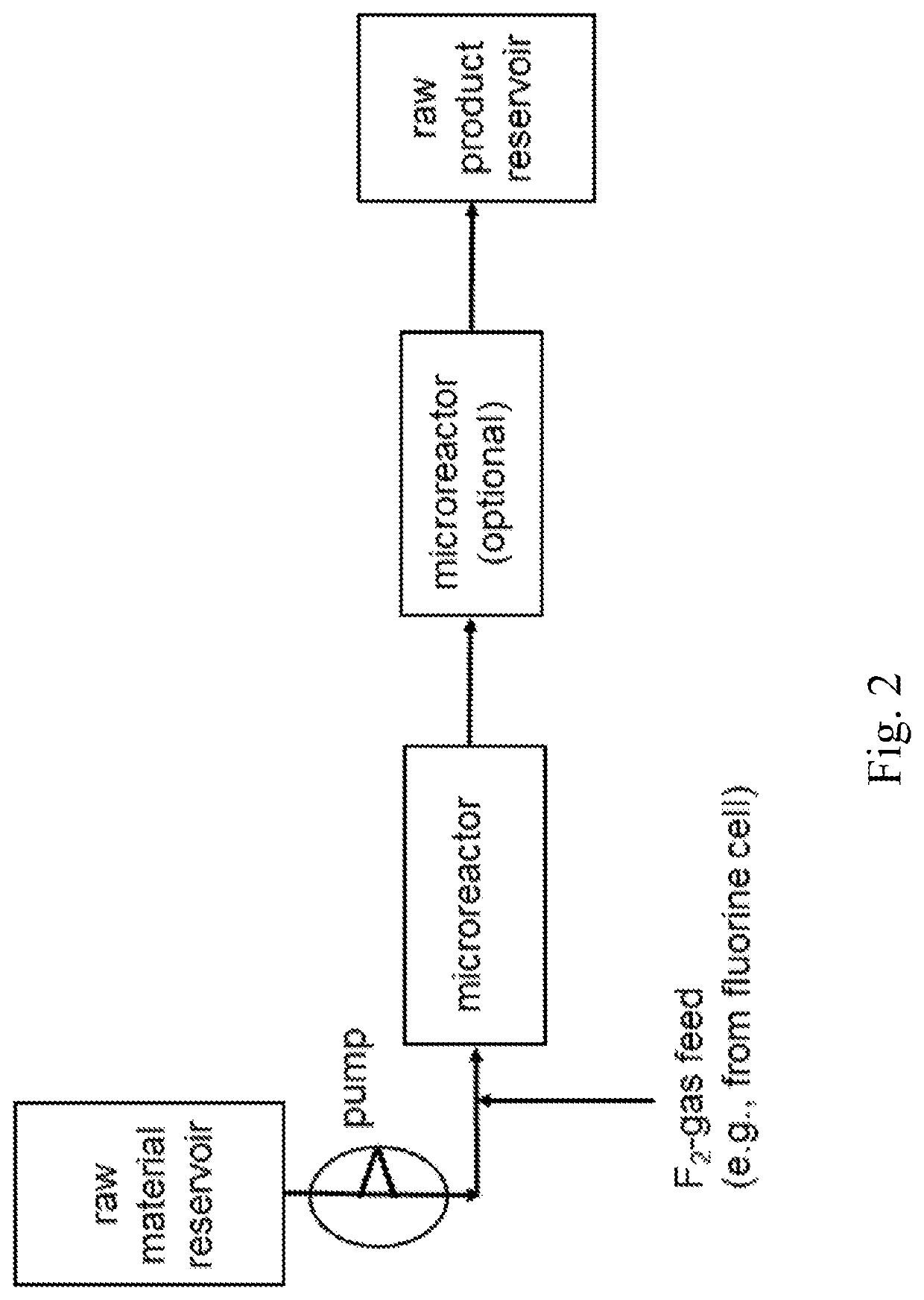
ActiveUS20210053895A1Simplify and reduce numberAvoid a lot of timeCarboxylic acid nitrile preparationOrganic compound preparationMicroreactorHigh concentration
The invention relates to a use of a fluorination gas, wherein the elemental fluorine (F2) is present in a high concentration, for example, in a concentration of elemental fluorine (F2), especially of equal to much higher than 15% or even 20% by volume (i.e., at least 15% or even 20% by volume), and to a process for the manufacture of a fluorinated benzene by direct fluorination employing a fluorination gas, wherein the elemental fluorine (F2) is present in a high concentration. The process of the invention is directed to the manufacture of a fluorinated benzene by direct fluorination. Especially the invention is of interest in the preparation of fluorinated benzene, final products and as well intermediates, for usage in agro-, pharma-, electronics-, catalyst, solvent and other functional chemical applications. The fluorination process of the invention may be performed batch-wise or in a continuous manner. If the process of the invention is performed batch-wise, a column (tower) reactor may be used. If the process of the invention is continuous a microreactor may be used. The invention is characterized in that the starting compound is benzene, and the fluorinated compound produced is a fluorinated benzene, preferably monofluorobenzene.
Owner:FUJIAN YONGJING TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com