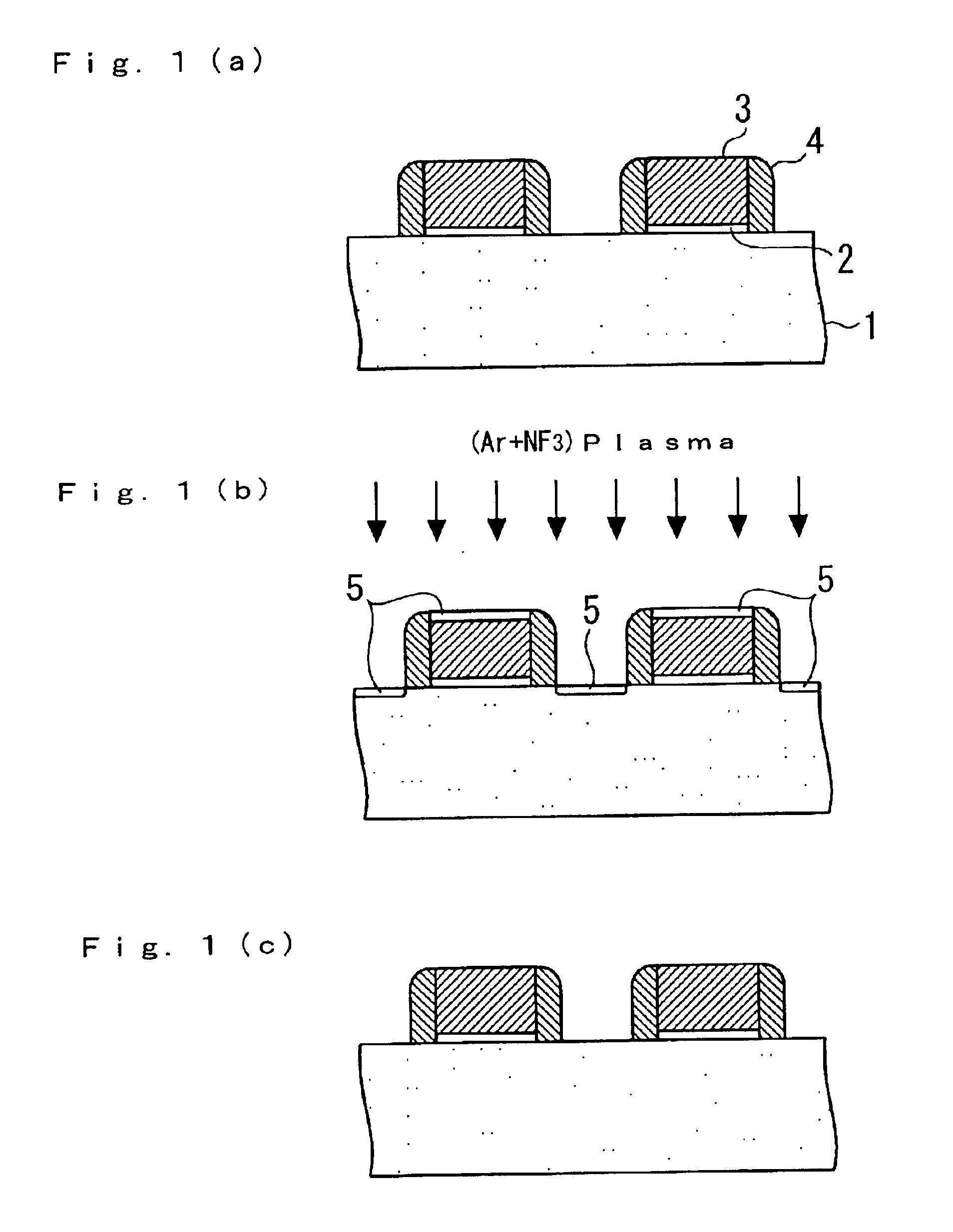
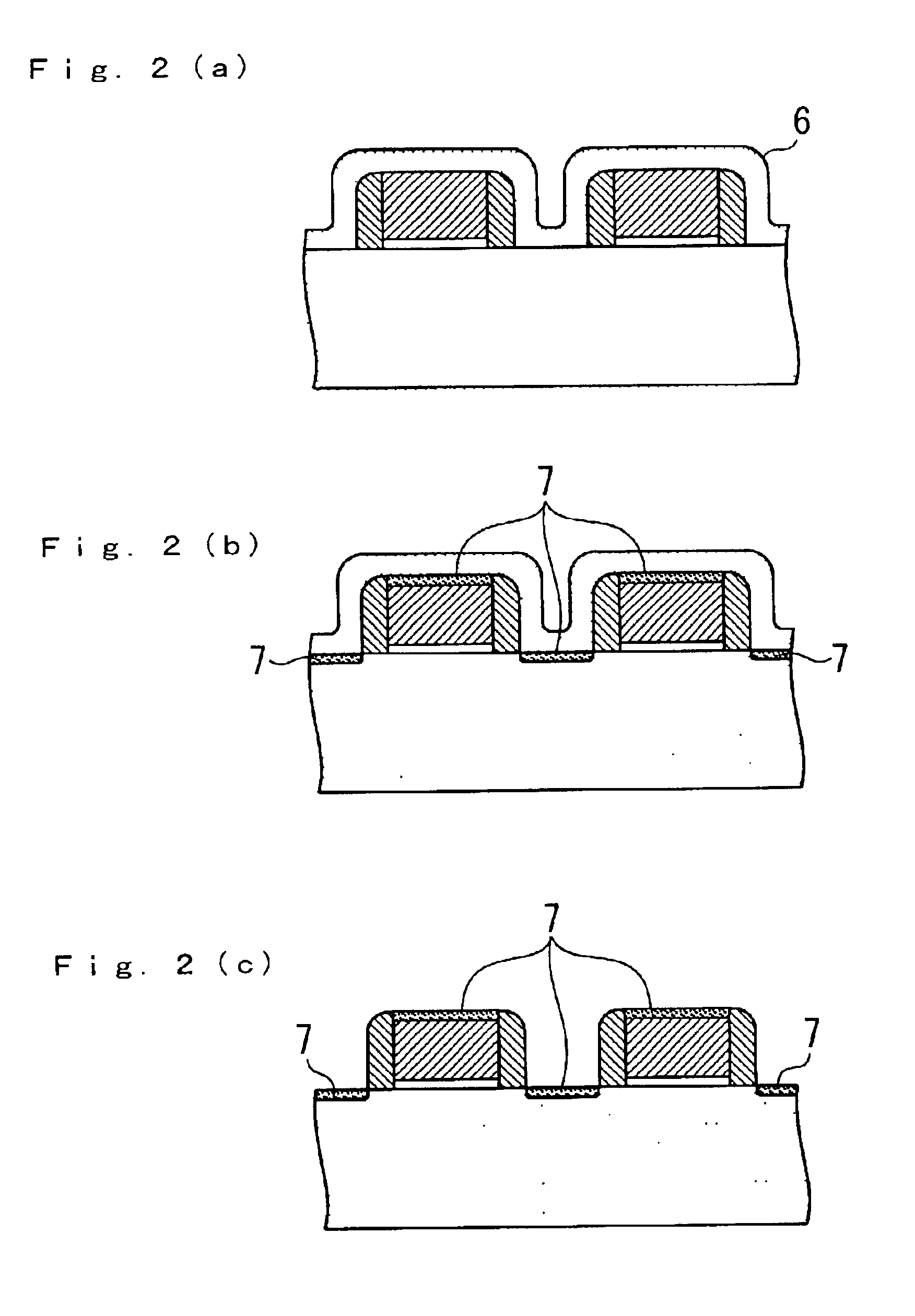
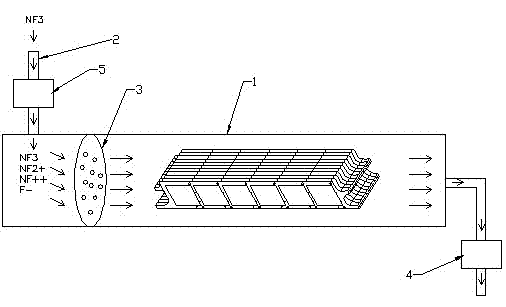
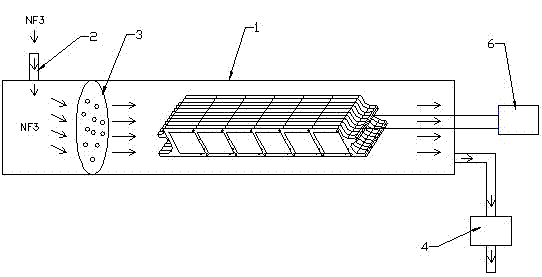
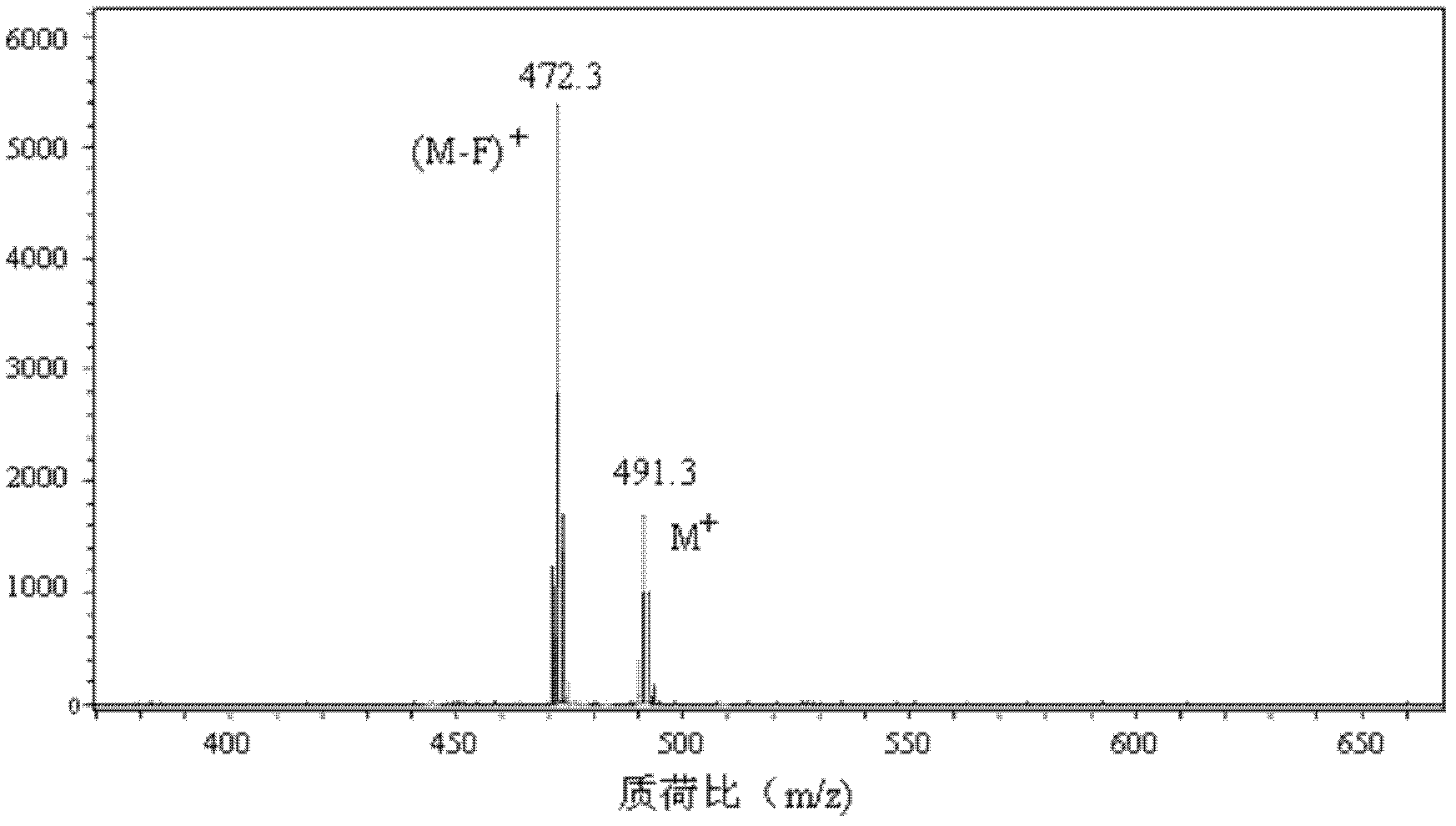Patents
Literature
324 results about "Nitrogen trifluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nitrogen trifluoride is the inorganic compound with the formula NF₃. This nitrogen-fluorine compound is a colorless, odorless, nonflammable gas. It finds increasing use as an etchant in microelectronics. Nitrogen trifluoride is an extremely strong greenhouse gas.
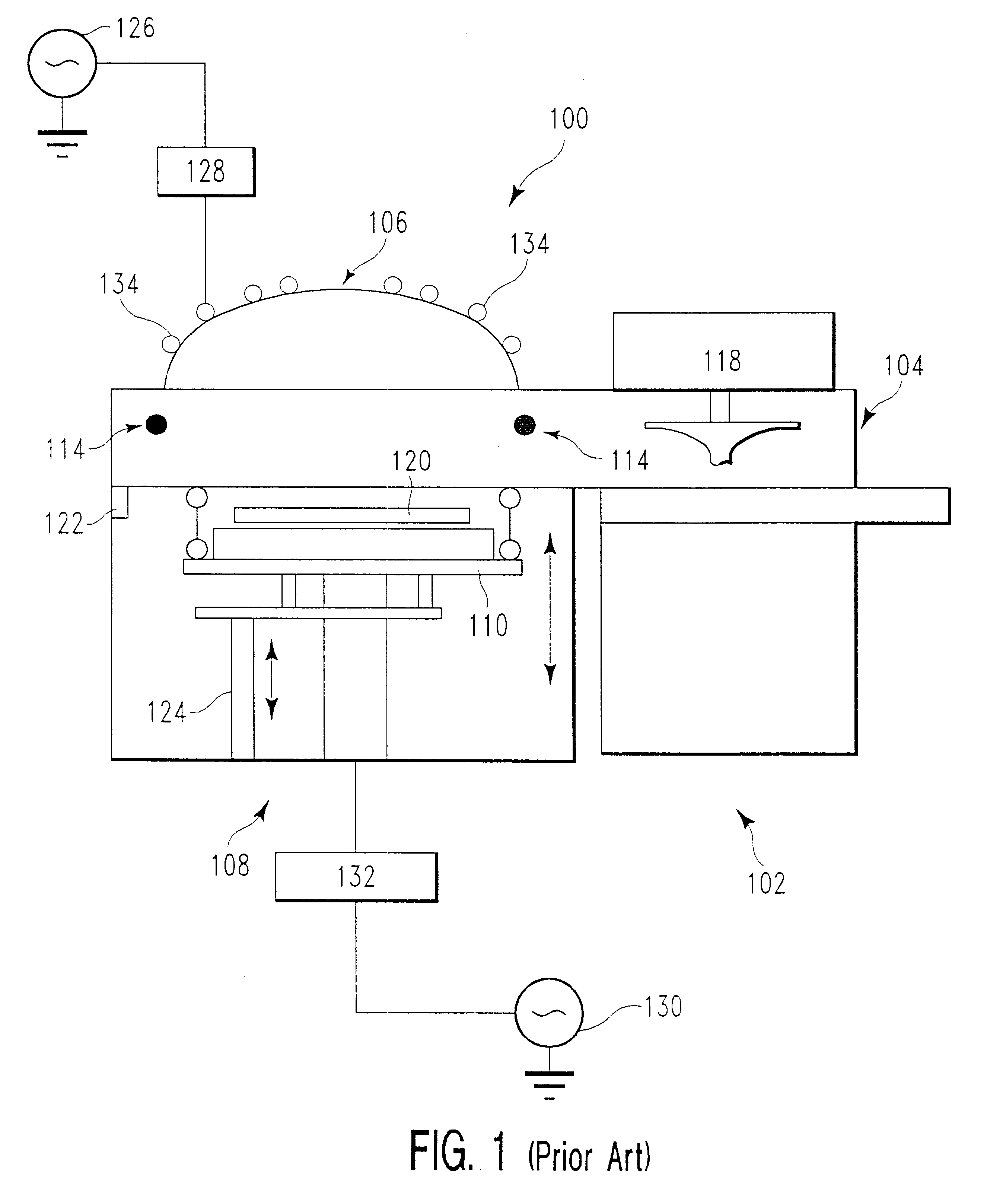
Method for activating reactive oxygen species for cleaning carbon-based film deposition
InactiveUS20090246399A1Liquid surface applicatorsElectrostatic cleaningNitrogenReactive oxygen species
A method of continuously forming carbon-based films on substrates includes: (i) forming a carbon-based film on a substrate in a reactor a pre-selected number of times; (ii) exciting an inert gas, an oxygen gas, and a nitrogen tri-fluoride gas to generate a plasma for cleaning; (iii) cleaning an inside of the reactor with the plasma after step (i) to remove particles accumulated during step (i) on the inside of the reactor.
Owner:ASM JAPAN
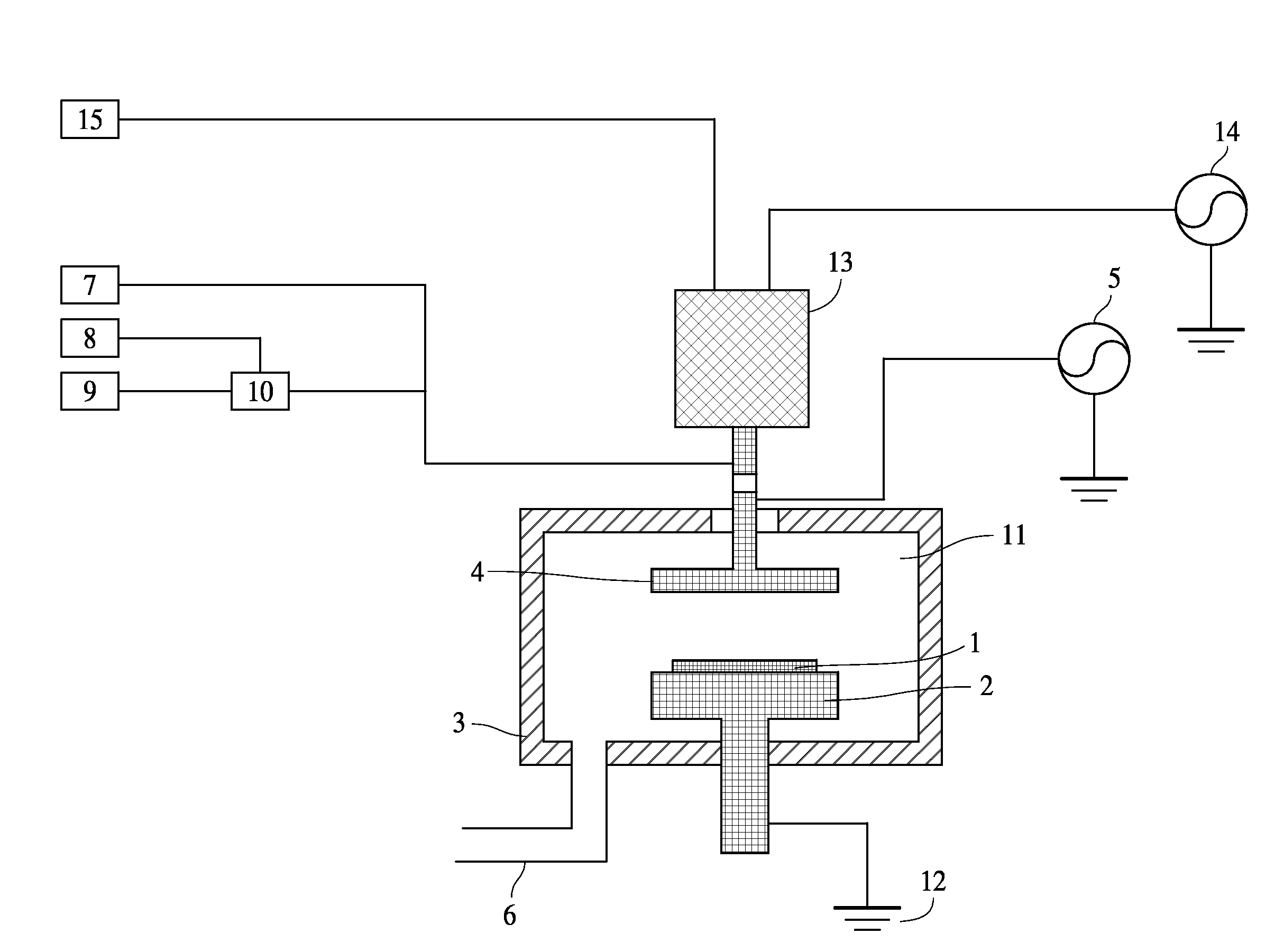
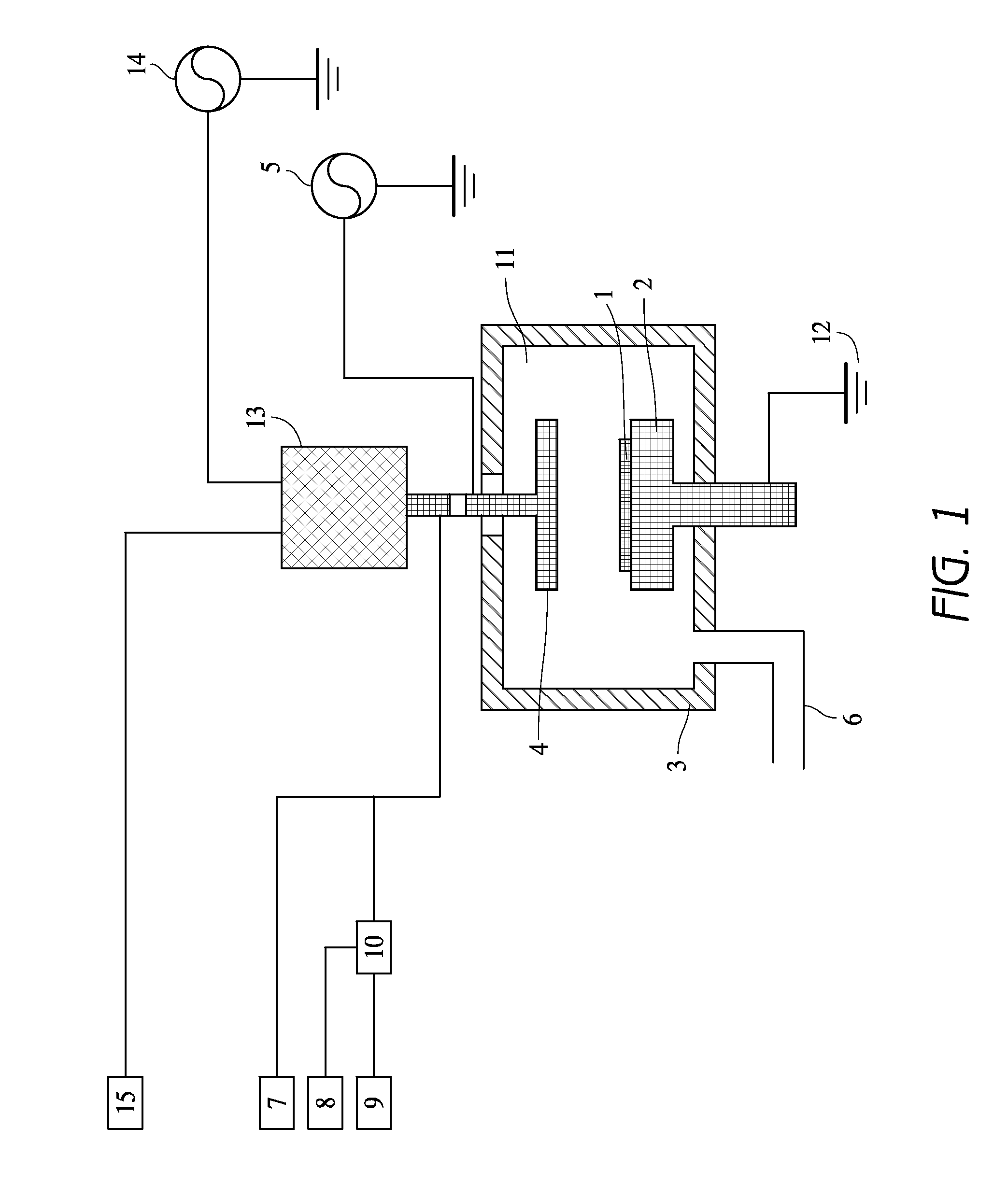
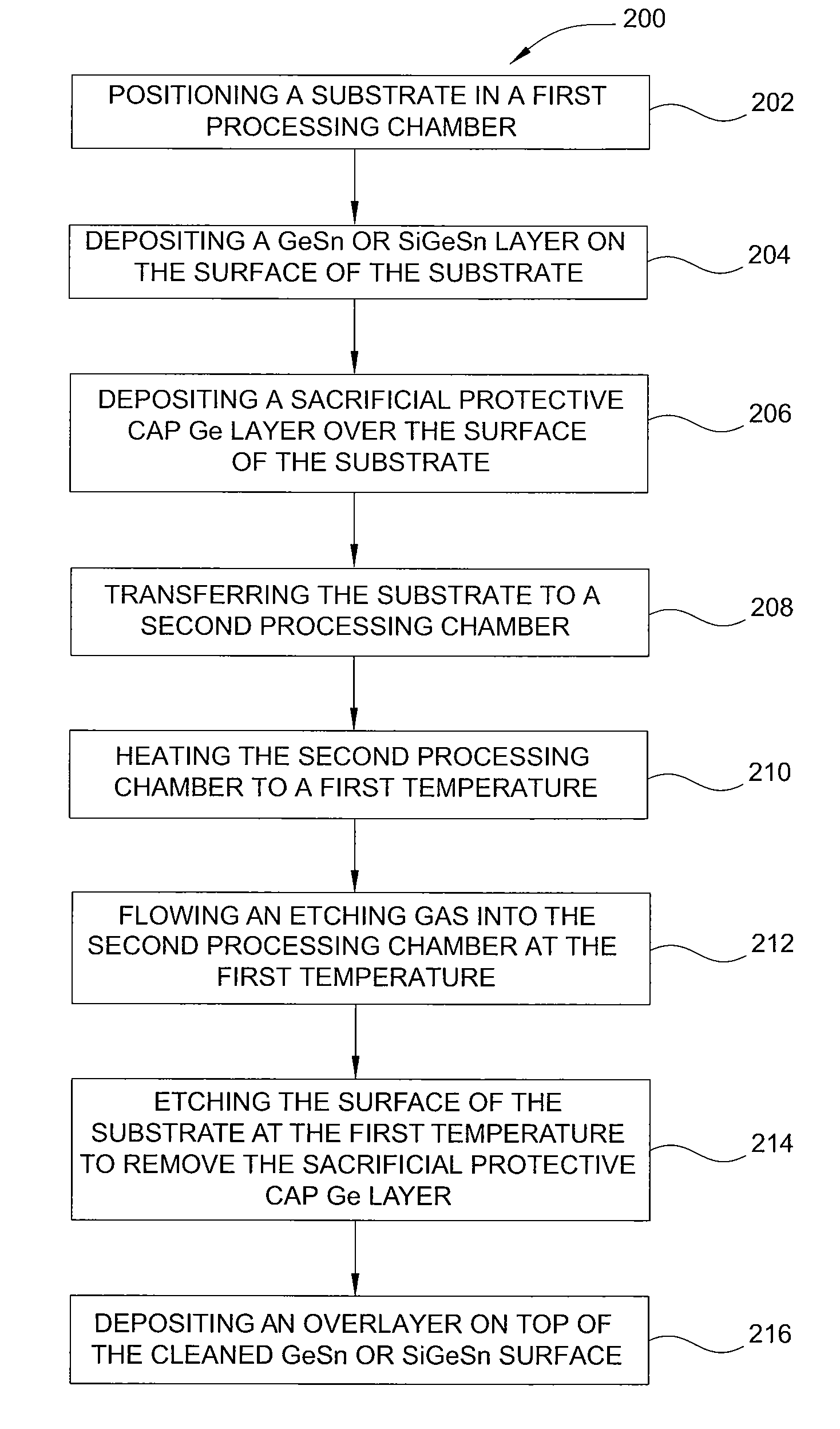
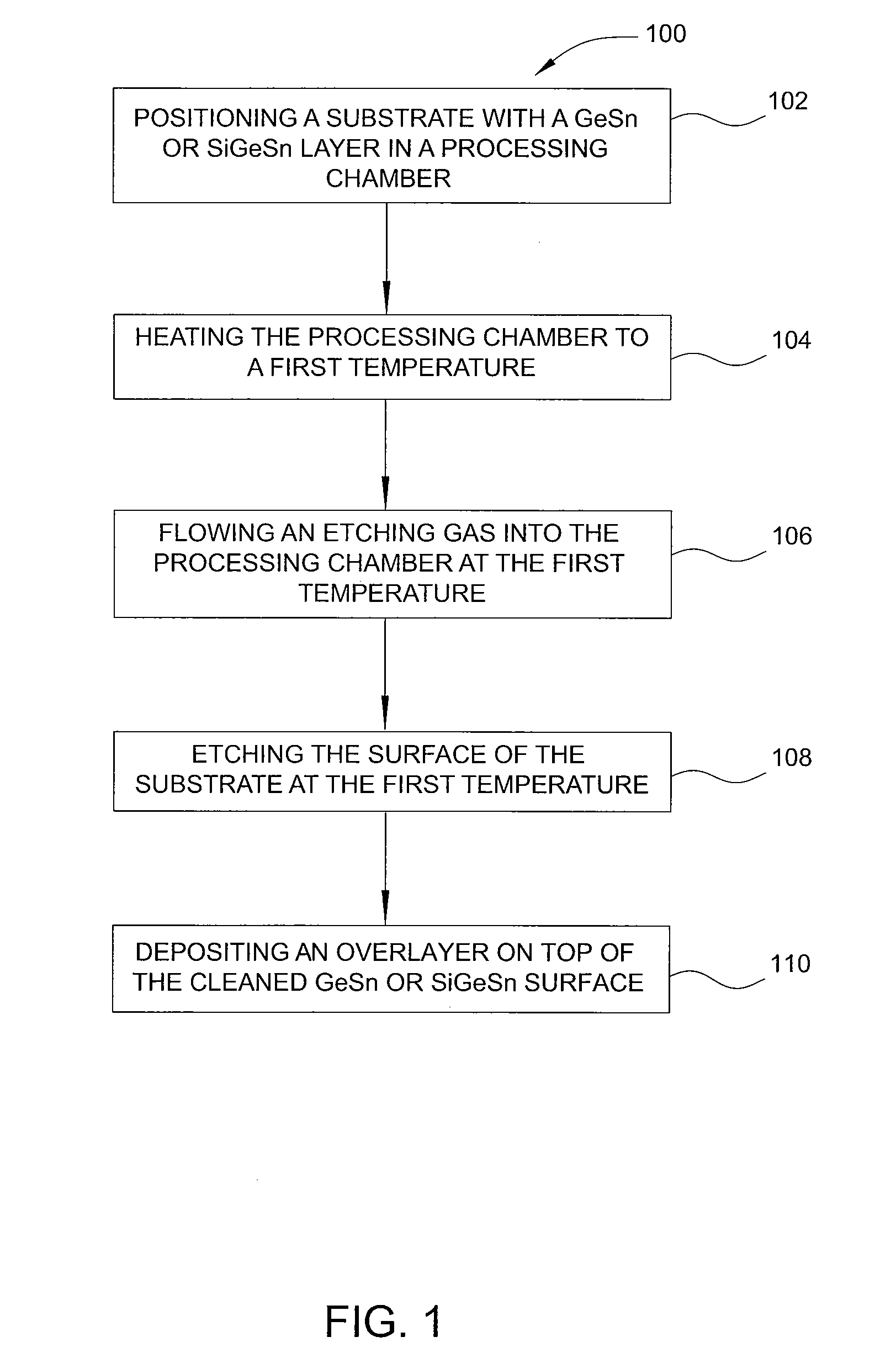
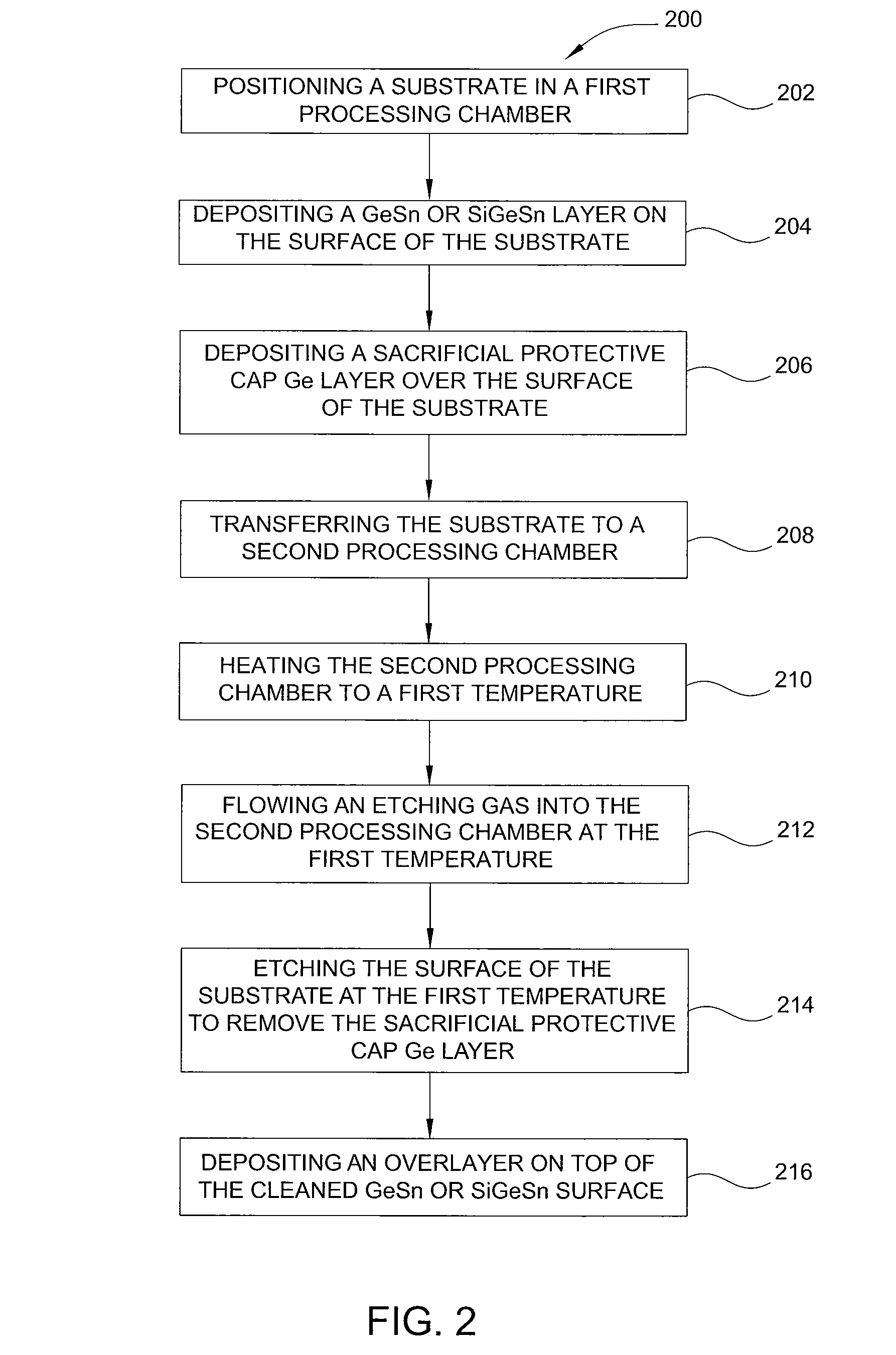
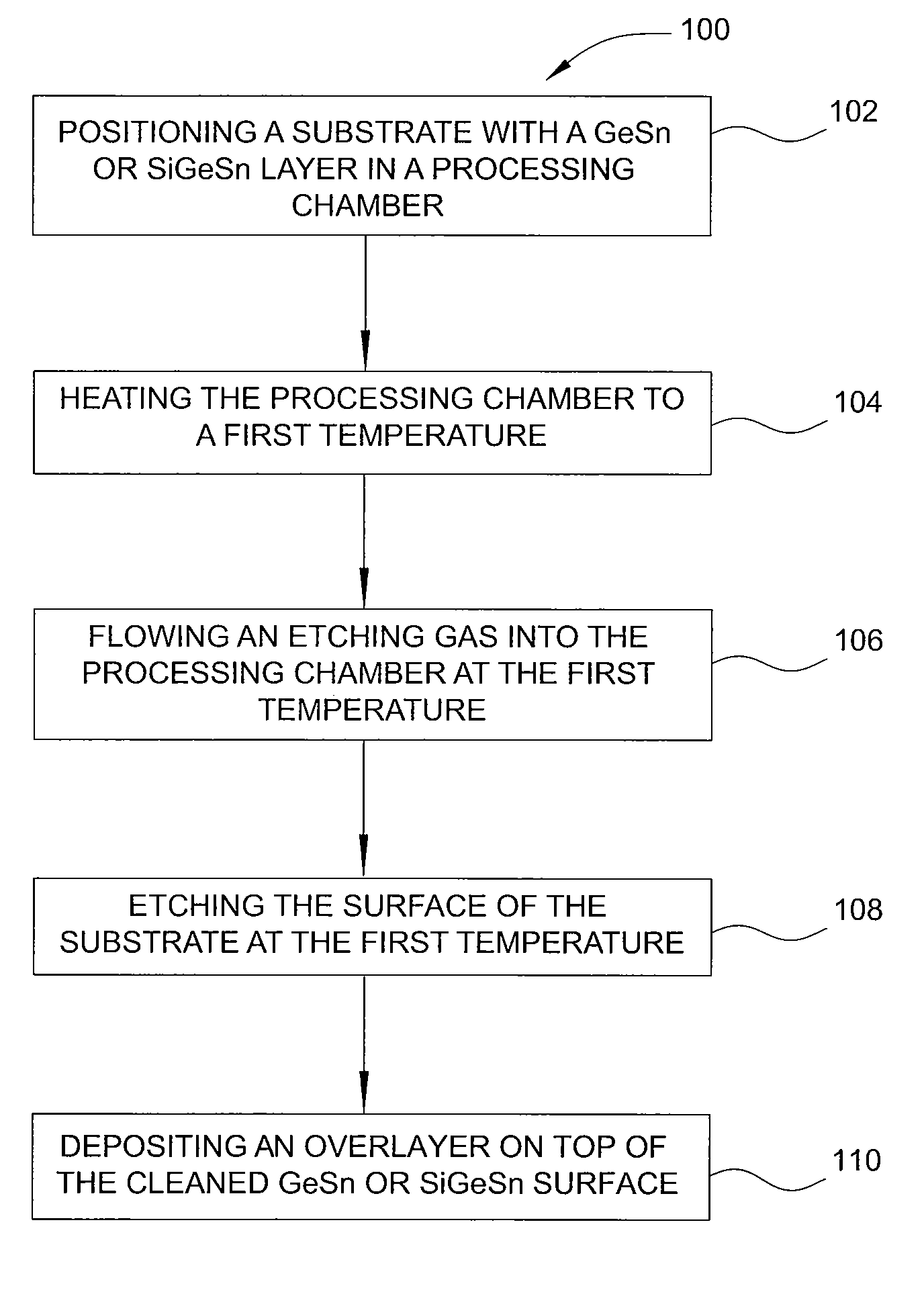
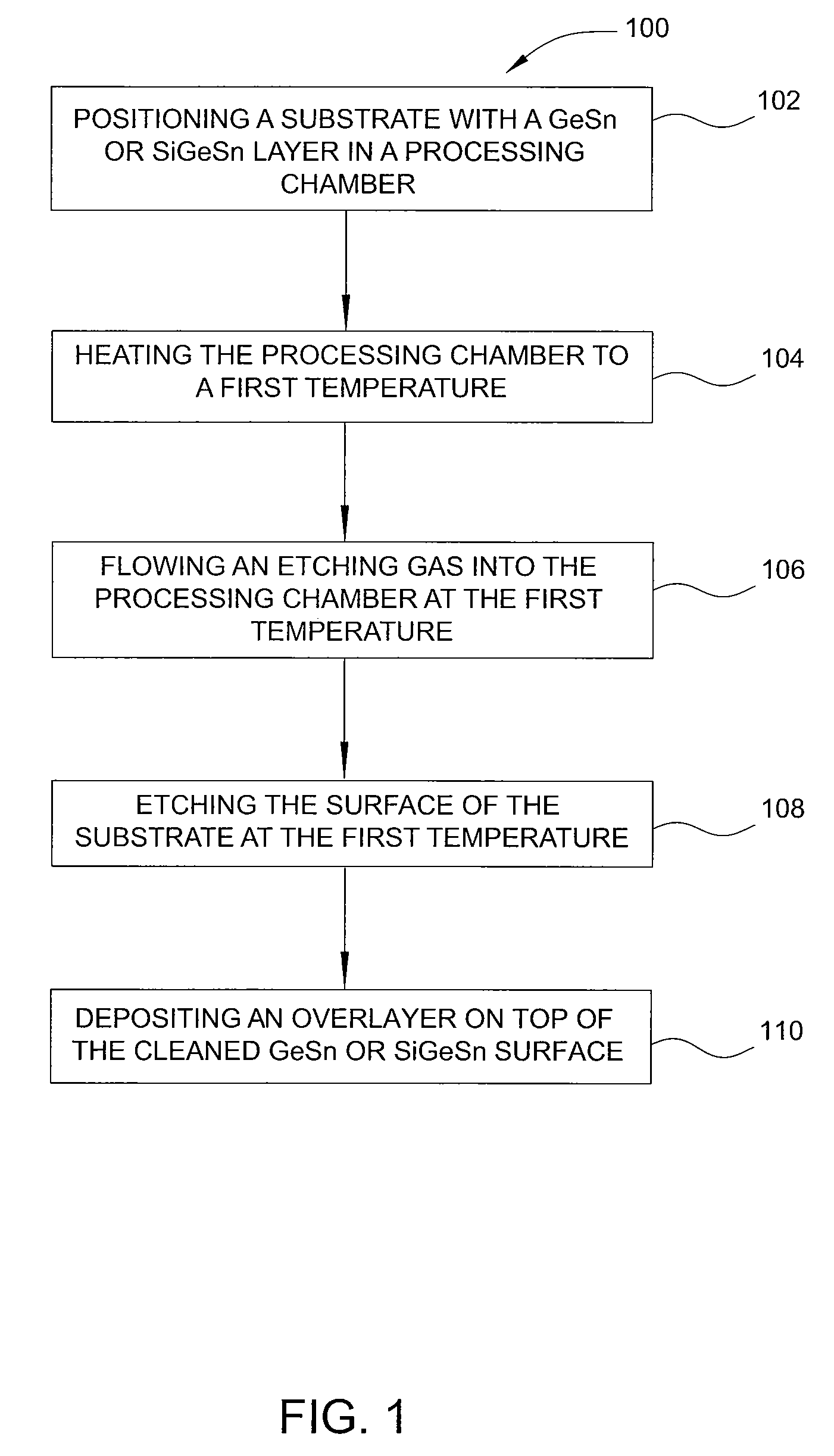
Method of epitaxial germanium tin alloy surface preparation
ActiveUS20130288480A1Increase temperatureStop the flowSemiconductor/solid-state device manufacturingPhotovoltaic energy generationEtchingRapid thermal annealing
Methods of preparing a clean surface of germanium tin or silicon germanium tin layers for subsequent deposition are provided. An overlayer of Ge, doped Ge, another GeSn or SiGeSn layer, a doped GeSn or SiGeSn layer, an insulator, or a metal can be deposited on a prepared GeSn or SiGeSn layer by positioning a substrate with an exposed germanium tin or silicon germanium tin layer in a processing chamber, heating the processing chamber and flowing a halide gas into the processing chamber to etch the surface of the substrate using either thermal or plasma assisted etching followed by depositing an overlayer on the substantially oxide free and contaminant free surface. Methods can also include the placement and etching of a sacrificial layer, a thermal clean using rapid thermal annealing, or a process in a plasma of nitrogen trifluoride and ammonia gas.
Owner:APPLIED MATERIALS INC
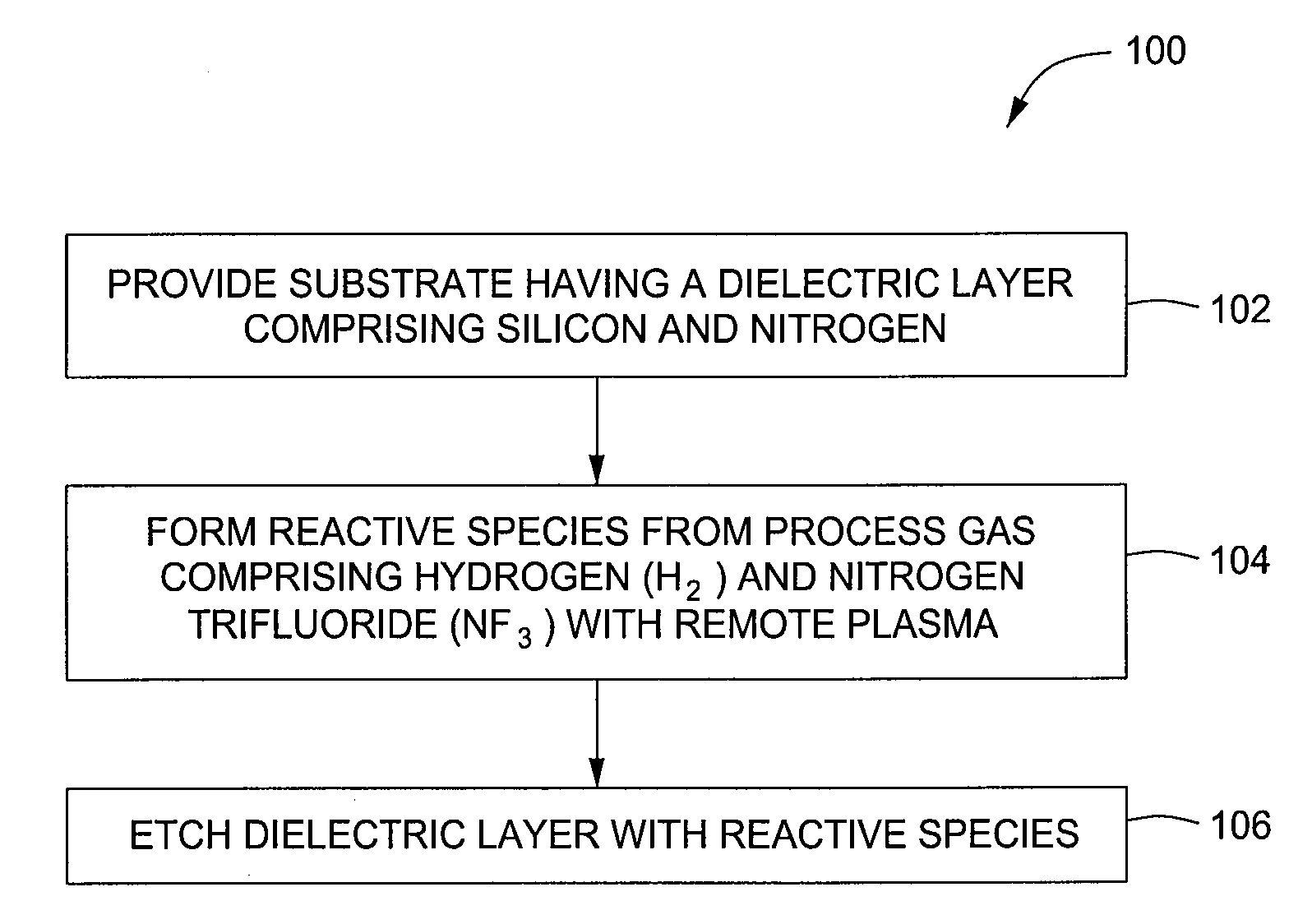
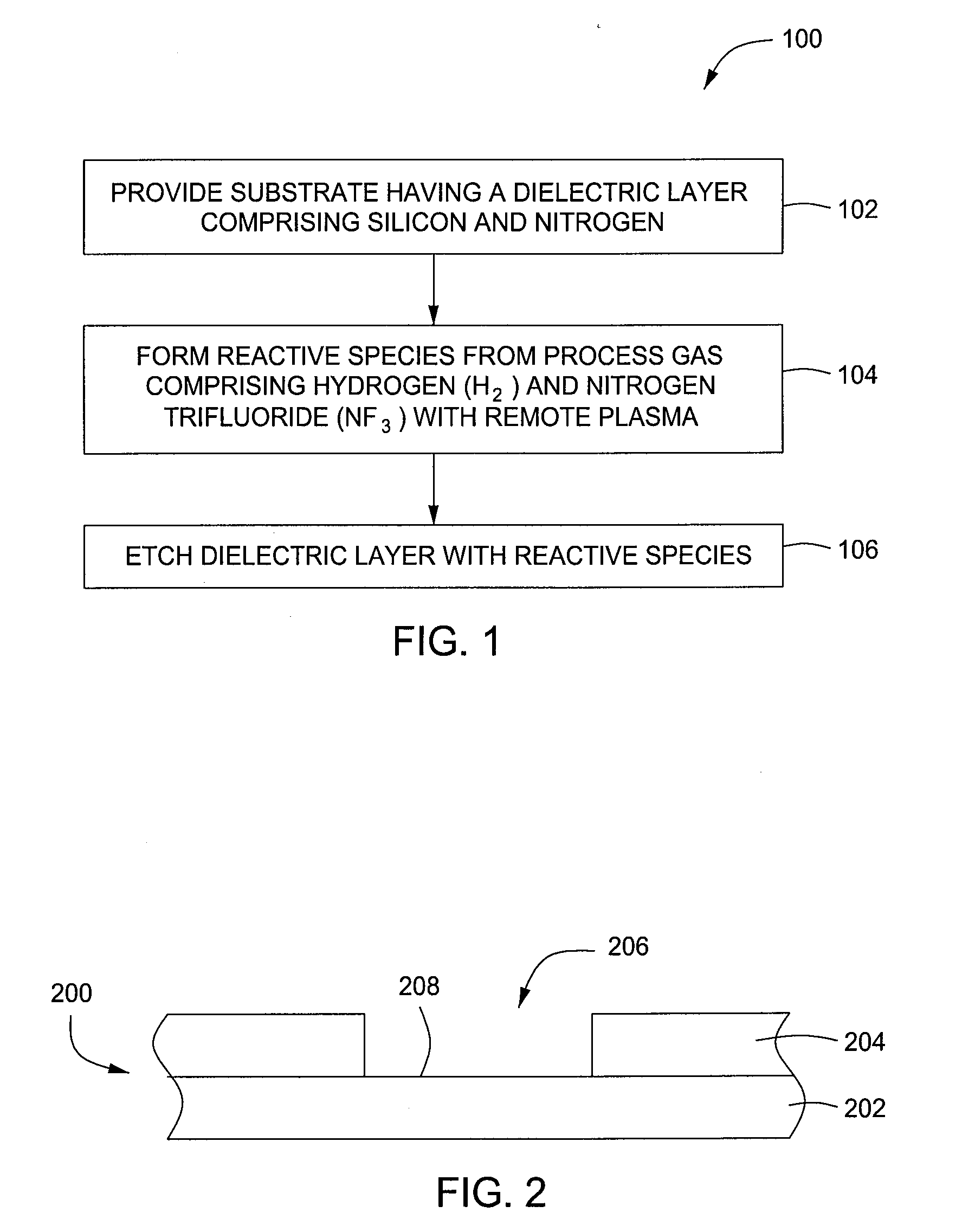
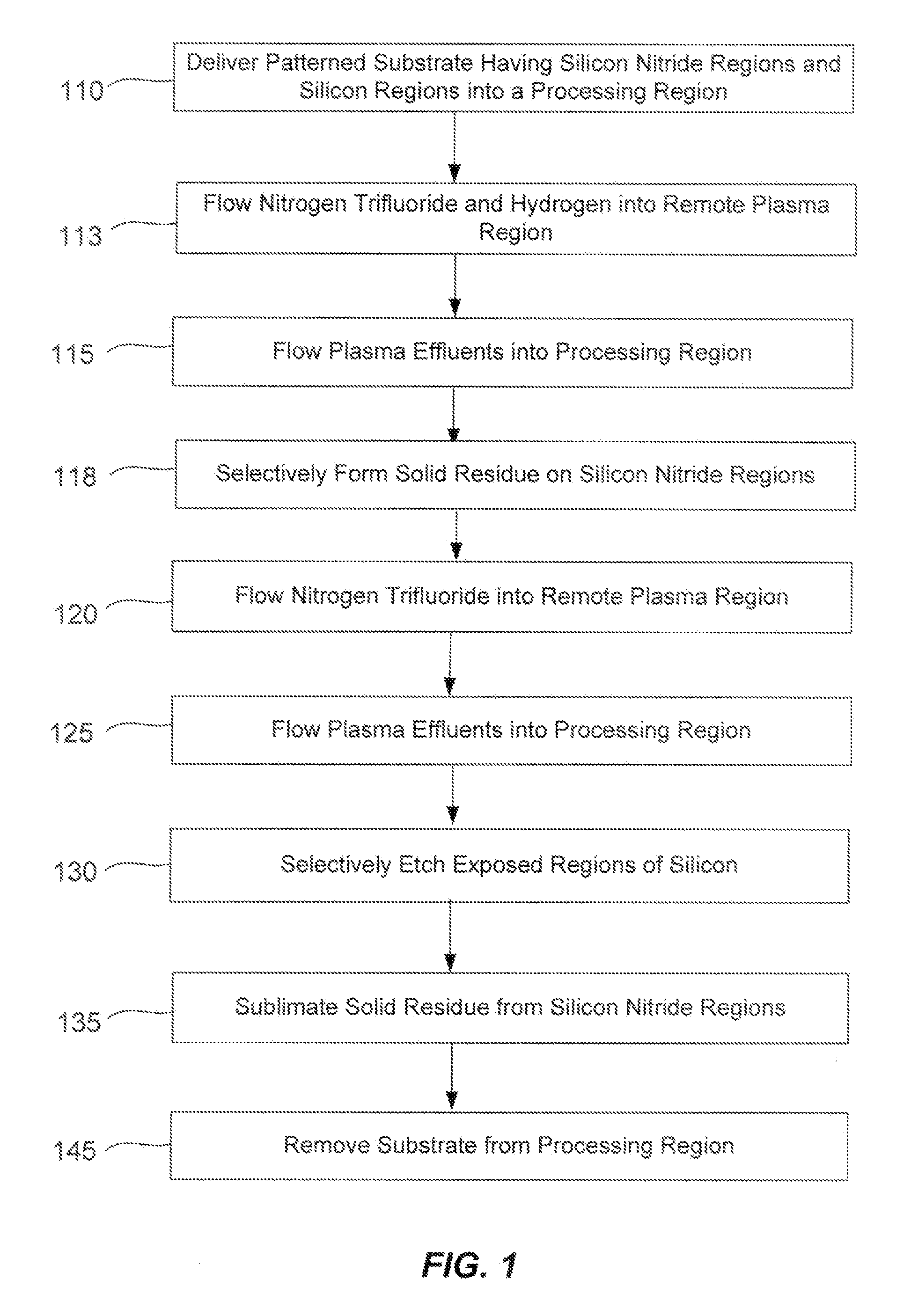
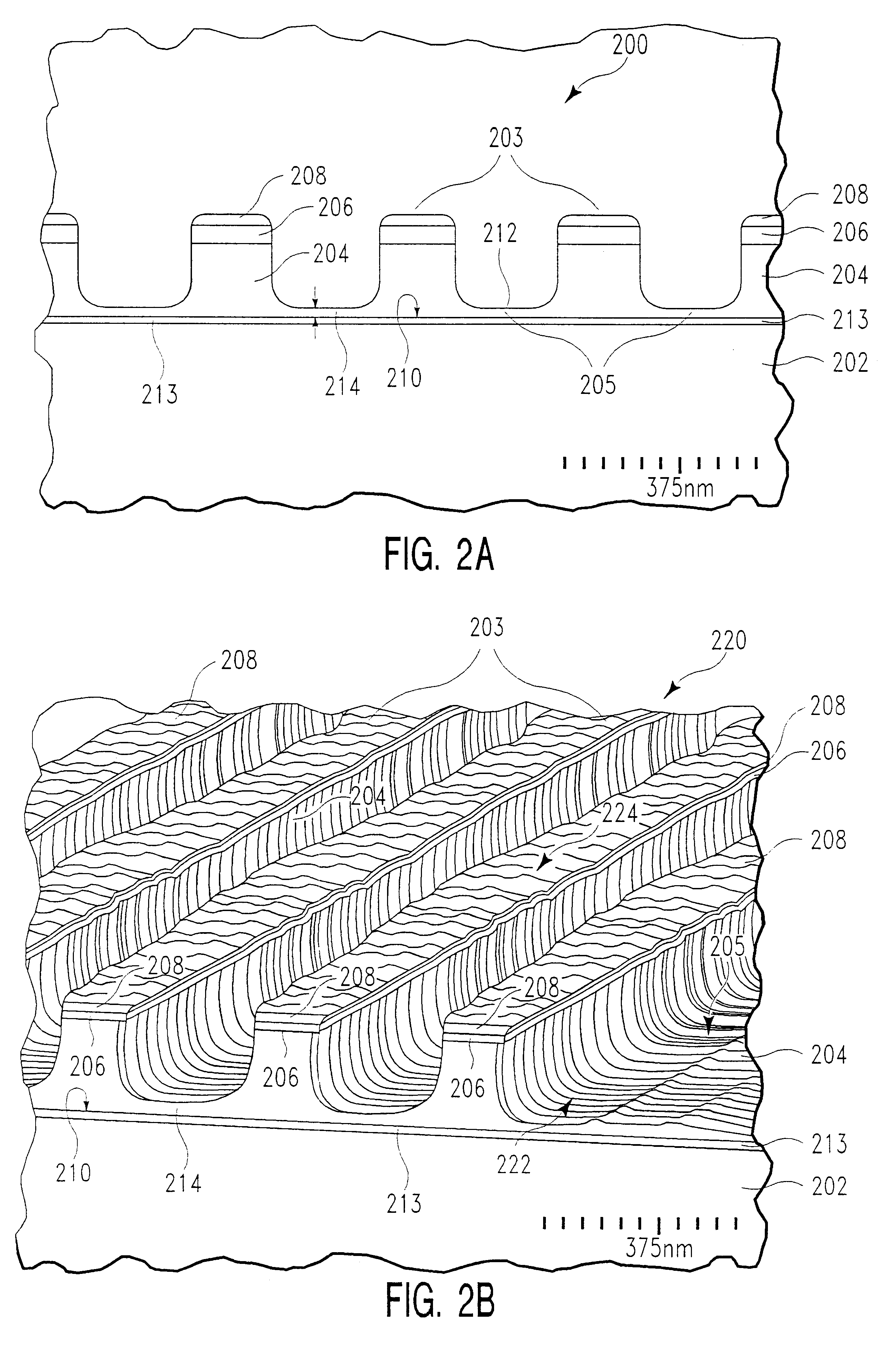
Selective etching of silicon nitride
InactiveUS20090104782A1Promotes selective oxidationSemiconductor/solid-state device manufacturingRemote plasmaHydrogen
Owner:APPLIED MATERIALS INC
Passivation layer formation by plasma clean process to reduce native oxide growth
ActiveUS20080160210A1Format be limitLighting and heating apparatusVacuum evaporation coatingAmmoniaSubstrate surface
Embodiments described herein provide methods for removing native oxide surfaces on substrates while simultaneously passivating the underlying substrate surface. In one embodiment, a method is provided which includes positioning a substrate containing an oxide layer within a processing chamber, adjusting a first temperature of the substrate to about 80° C. or less, generating a cleaning plasma from a gas mixture within the processing chamber, such that the gas mixture contains ammonia and nitrogen trifluoride having an NH3 / NF3 molar ratio of about 10 or greater, and condensing the cleaning plasma onto the substrate. A thin film, containing ammonium hexafluorosilicate, is formed in part, from the native oxide during a plasma clean process. The method further includes heating the substrate to a second temperature of about 100° C. or greater within the processing chamber while removing the thin film from the substrate and forming a passivation surface thereon.
Owner:APPLIED MATERIALS INC
Silicon nitride etching methods
ActiveUS7288482B2Semiconductor/solid-state device manufacturingSulfur hexafluorideOctafluorocyclobutane
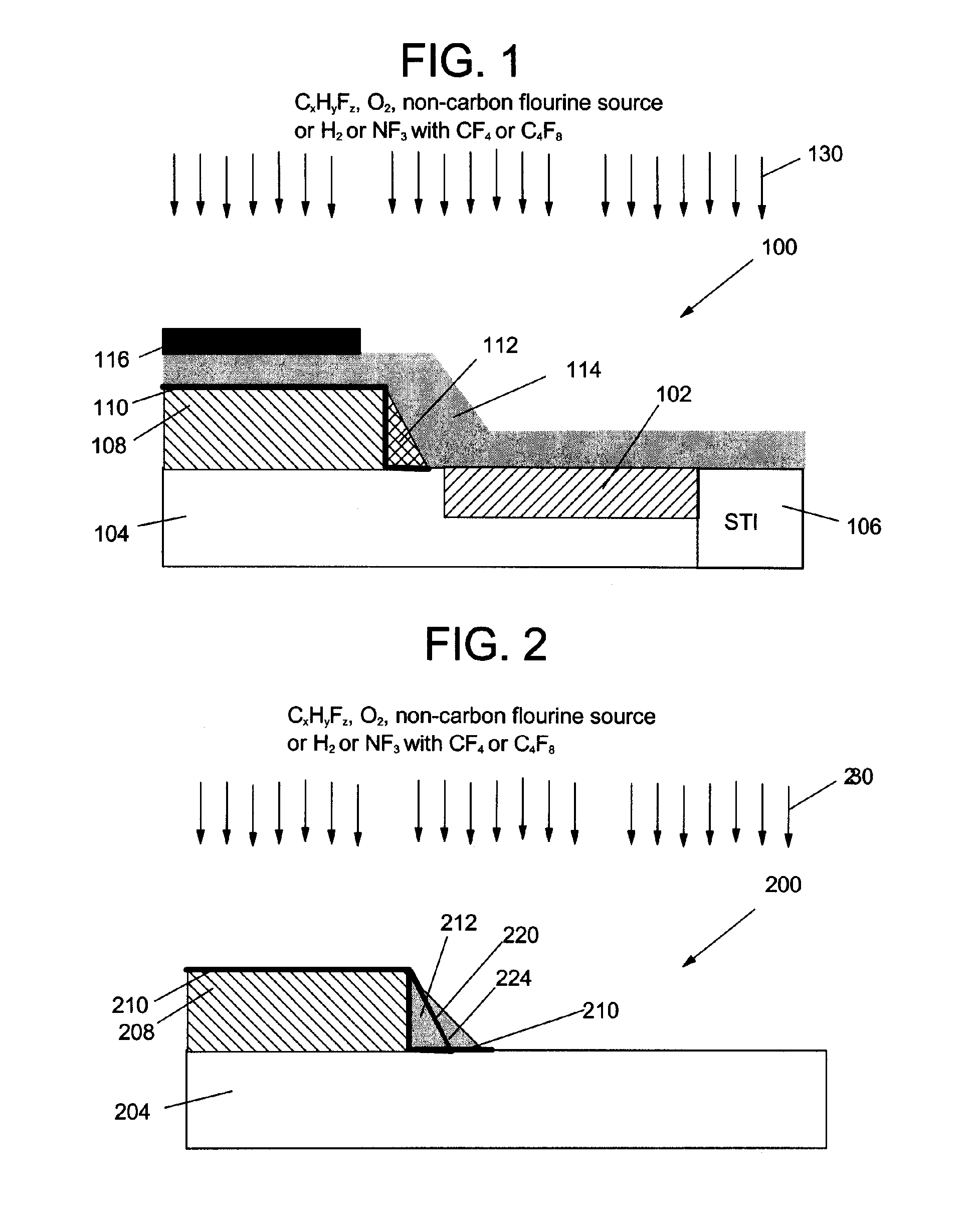
Methods of etching silicon nitride material, and more particularly, etching nitride selective to silicon dioxide or silicide, are disclosed. The methods include exposing a substrate having silicon nitride thereon to a plasma including at least one fluorohydrocarbon and a non-carbon containing fluorine source such as sulfur hexafluoride (SF6). The plasma may also include oxygen (O2) and the fluorohydrocarbons may include at least one of: trifluoromethane (CHF3), difluoromethane (CH2F2), and methyl fluoride (CH3F). In an alternative embodiment, the plasma includes one of hydrogen (H2) and nitrogen trifluoride (NF3) and one of tetrafluoromethane (CF4) and octafluorocyclobutane (C4F8). The methods are preferably carried out using a low bias voltage, e.g. <100 V.
Owner:IBM CORP +1
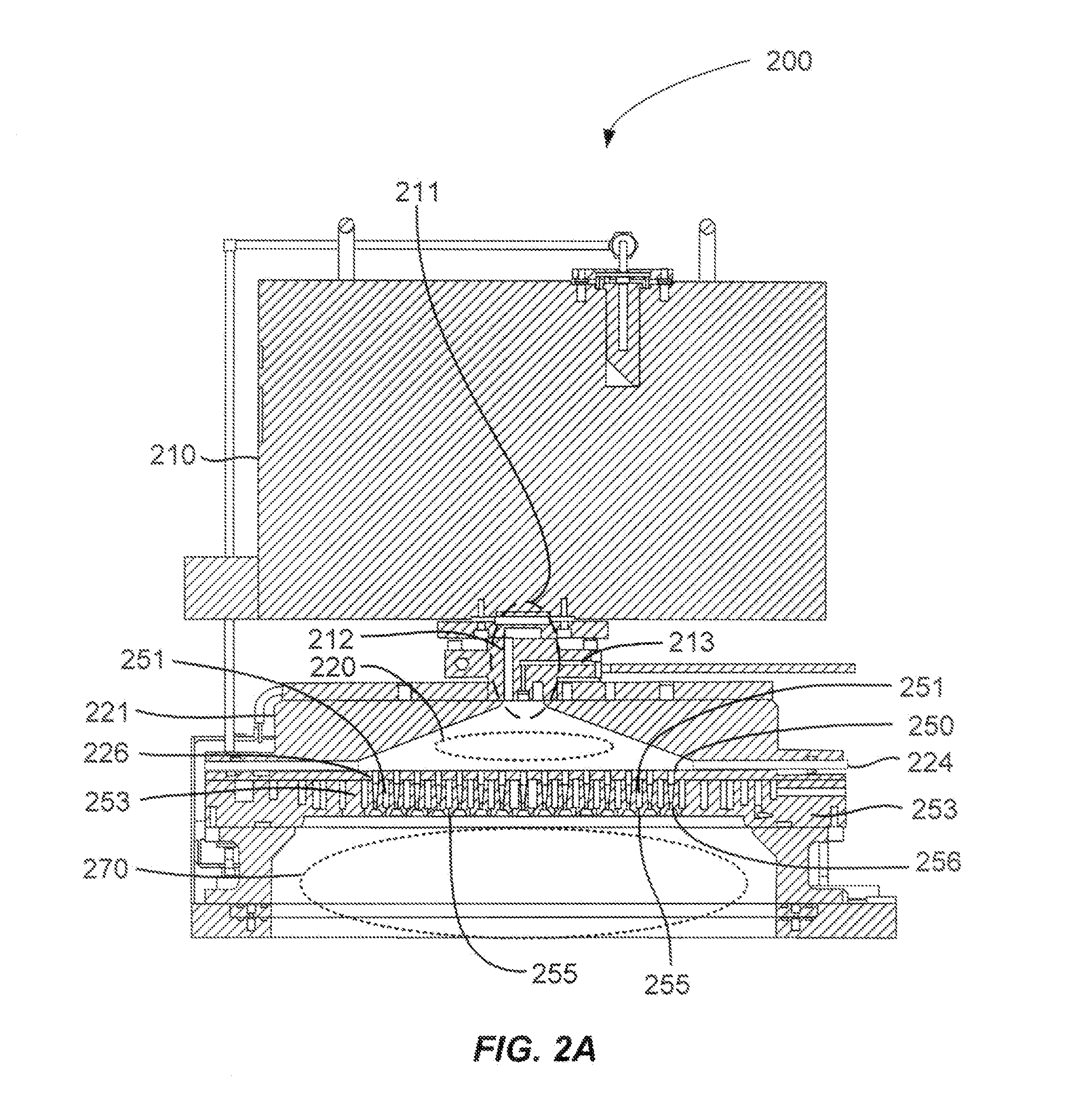
Nf3/h2 remote plasma process with high etch selectivity of psg/bpsg over thermal oxide and low density surface defects
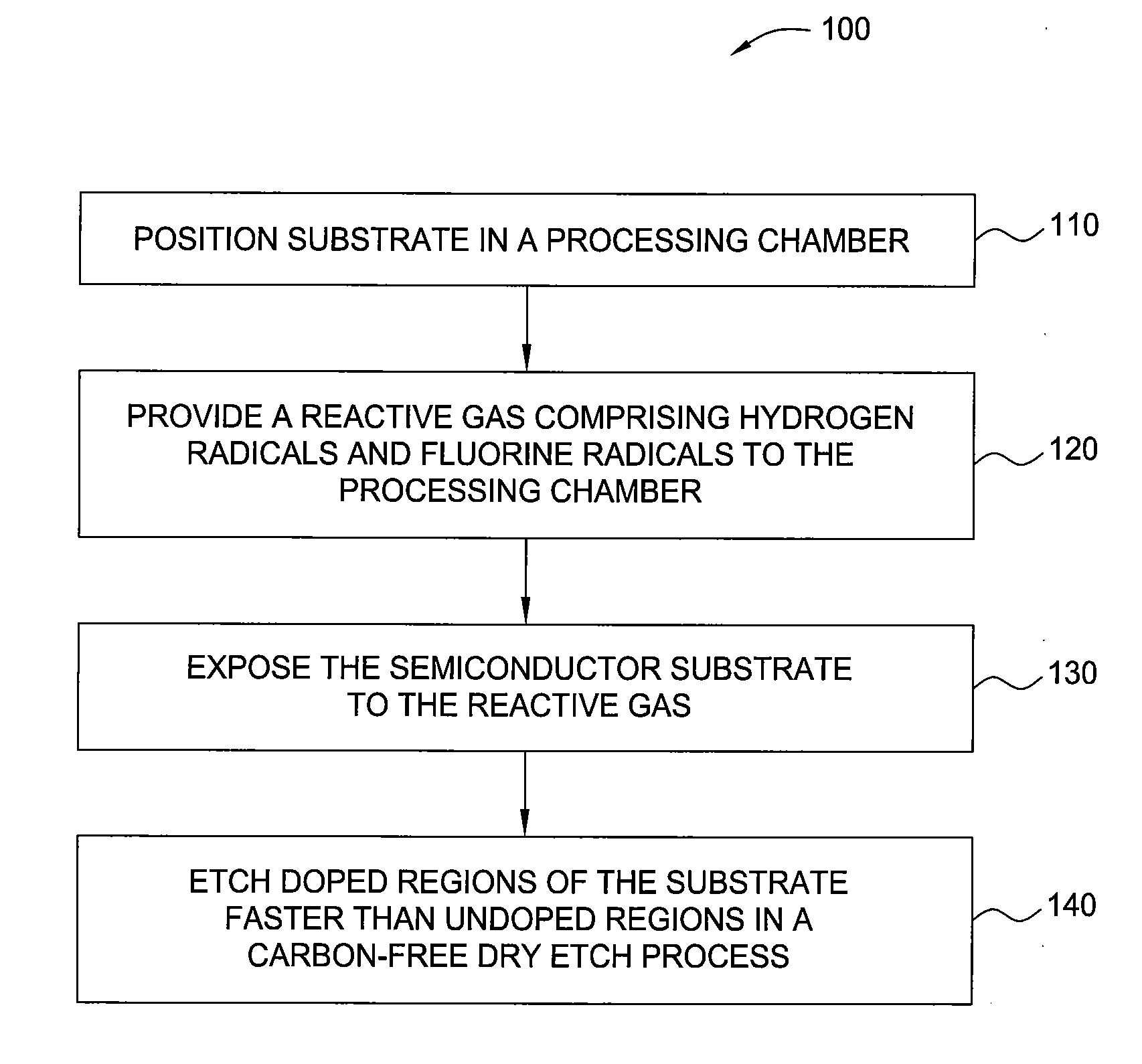
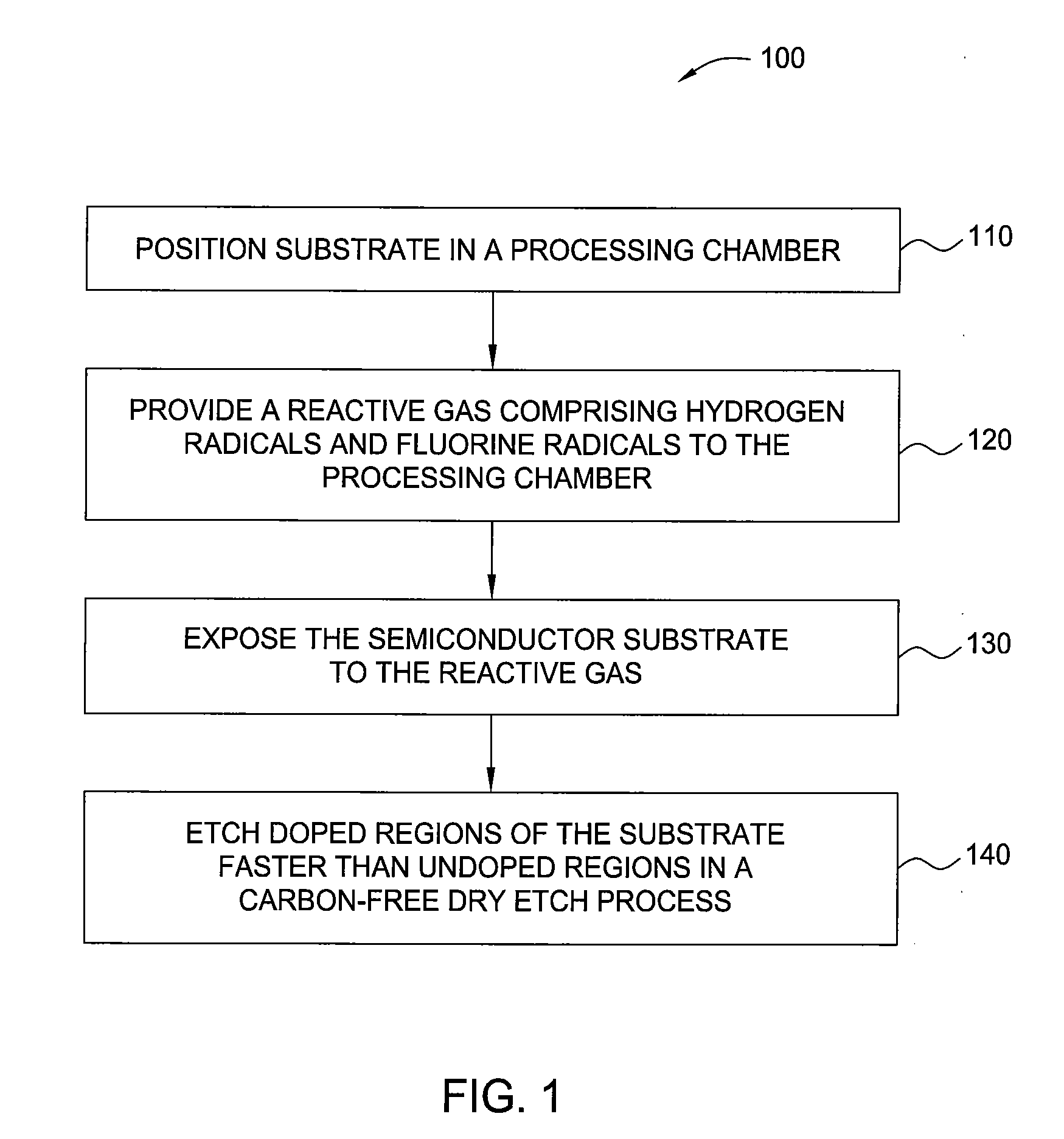
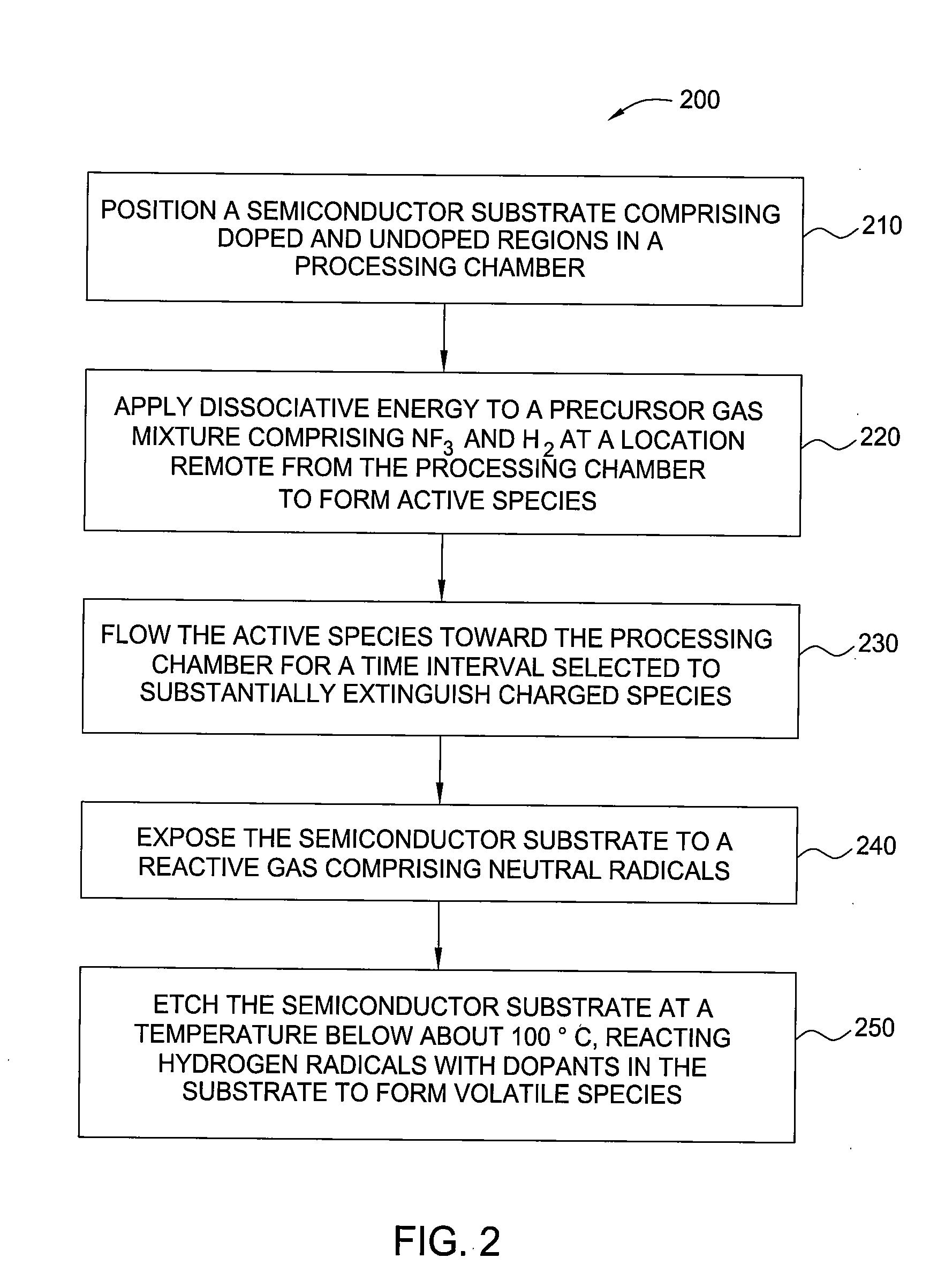
InactiveUS20100099263A1Fast etchElectric discharge tubesSemiconductor/solid-state device manufacturingHydrogenRemote plasma
A method and apparatus for selectively etching doped semiconductor oxides faster than undoped oxides. The method comprises applying dissociative energy to a mixture of nitrogen trifluoride and hydrogen gas remotely, flowing the activated gas toward a processing chamber to allow time for charged species to be extinguished, and applying the activated gas to the substrate. Reducing the ratio of hydrogen to nitrogen trifluoride increases etch selectivity. A similar process may be used to smooth surface defects in a silicon surface.
Owner:APPLIED MATERIALS INC
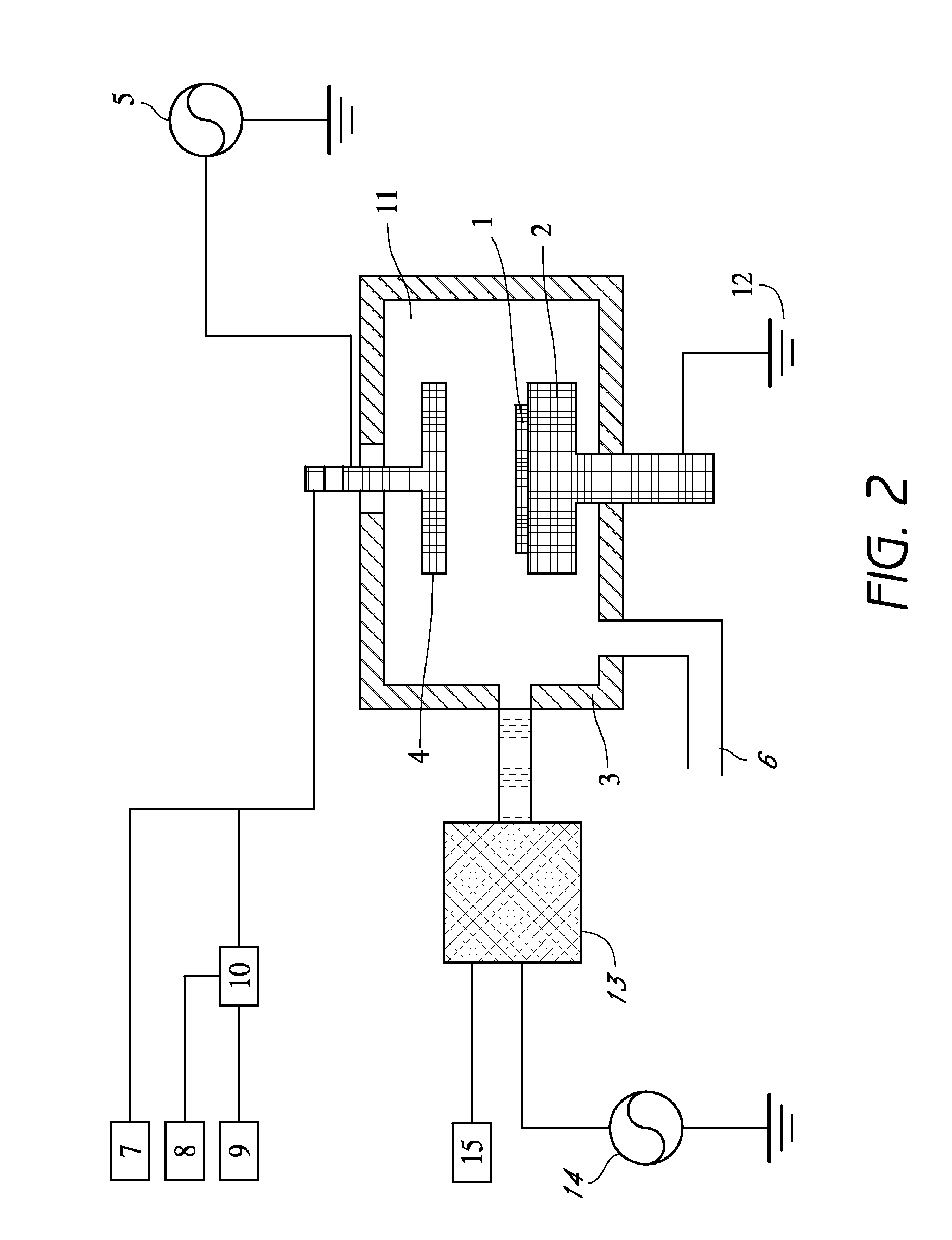
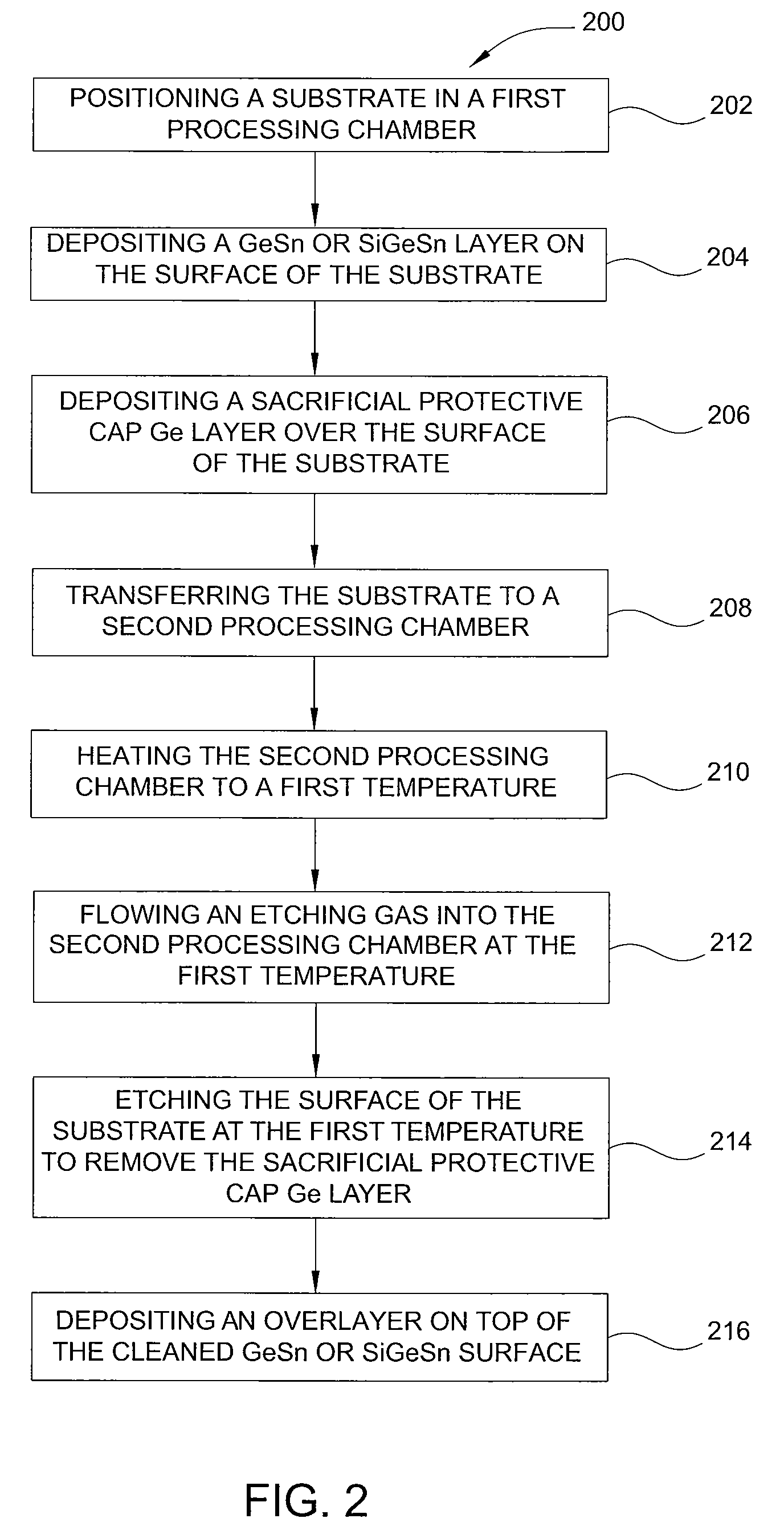
Method of epitaxial germanium tin alloy surface preparation
Methods of preparing a clean surface of germanium tin or silicon germanium tin layers for subsequent deposition are provided. An overlayer of Ge, doped Ge, another GeSn or SiGeSn layer, a doped GeSn or SiGeSn layer, an insulator, or a metal can be deposited on a prepared GeSn or SiGeSn layer by positioning a substrate with an exposed germanium tin or silicon germanium tin layer in a processing chamber, heating the processing chamber and flowing a halide gas into the processing chamber to etch the surface of the substrate using either thermal or plasma assisted etching followed by depositing an overlayer on the substantially oxide free and contaminant free surface. Methods can also include the placement and etching of a sacrificial layer, a thermal clean using rapid thermal annealing, or a process in a plasma of nitrogen trifluoride and ammonia gas.
Owner:APPLIED MATERIALS INC
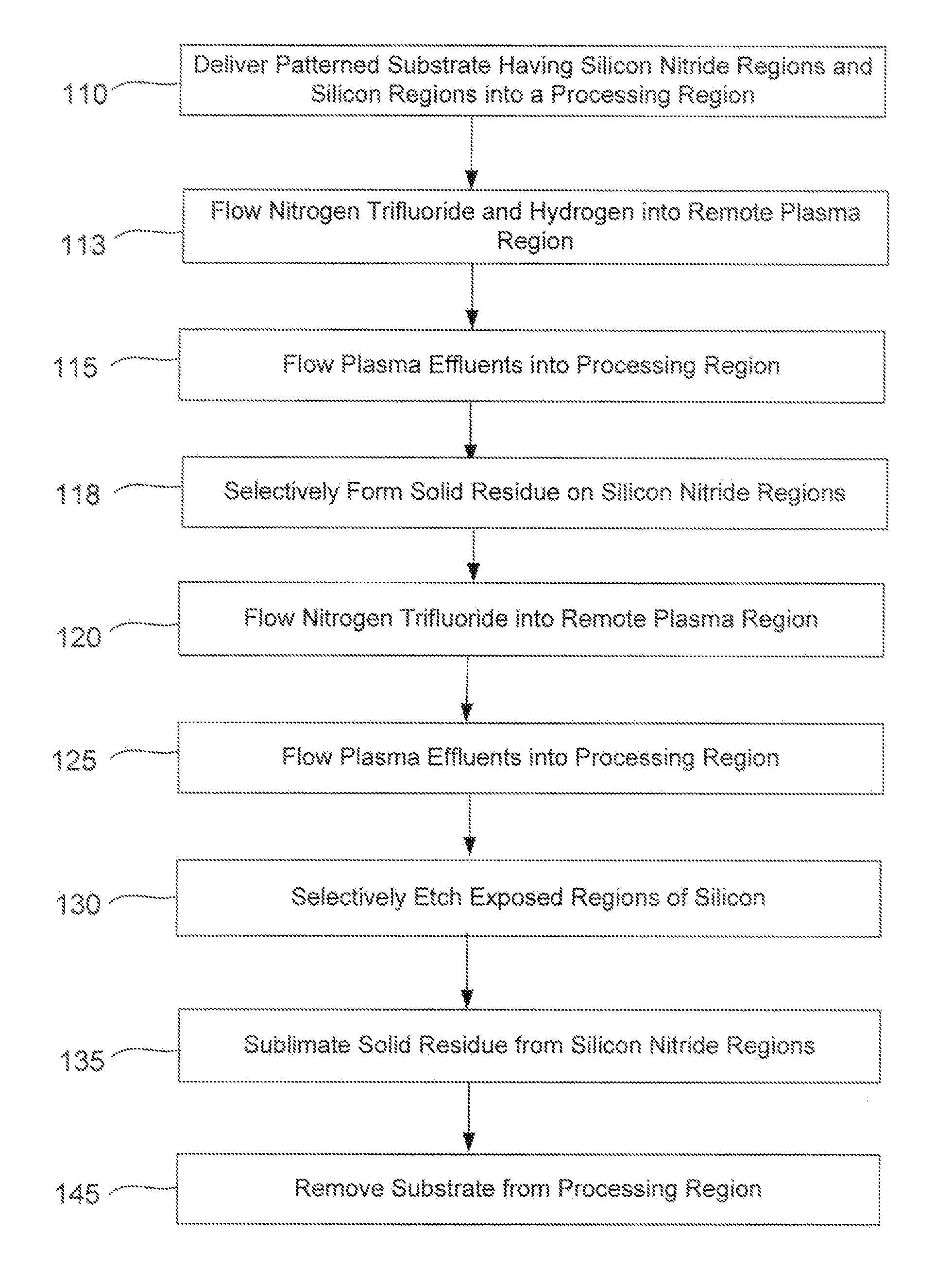
Selective suppression of dry-etch rate of materials containing both silicon and nitrogen
InactiveUS20130059440A1Inhibit etch rateImprove etch selectivityElectric discharge tubesSemiconductor/solid-state device manufacturingRemote plasmaHydrogen
A method of suppressing the etch rate for exposed silicon-and-nitrogen-containing material on patterned heterogeneous structures is described and includes a two stage remote plasma etch. The etch selectivity of silicon relative to silicon nitride and other silicon-and-nitrogen-containing material is increased using the method. The first stage of the remote plasma etch reacts plasma effluents with the patterned heterogeneous structures to form protective solid by-product on the silicon-and-nitrogen-containing material. The plasma effluents of the first stage are formed from a remote plasma of a combination of precursors, including nitrogen trifluoride and hydrogen (H2). The second stage of the remote plasma etch also reacts plasma effluents with the patterned heterogeneous structures to selectively remove material which lacks the protective solid by-product. The plasma effluents of the second stage are formed from a remote plasma of a fluorine-containing precursor.
Owner:APPLIED MATERIALS INC
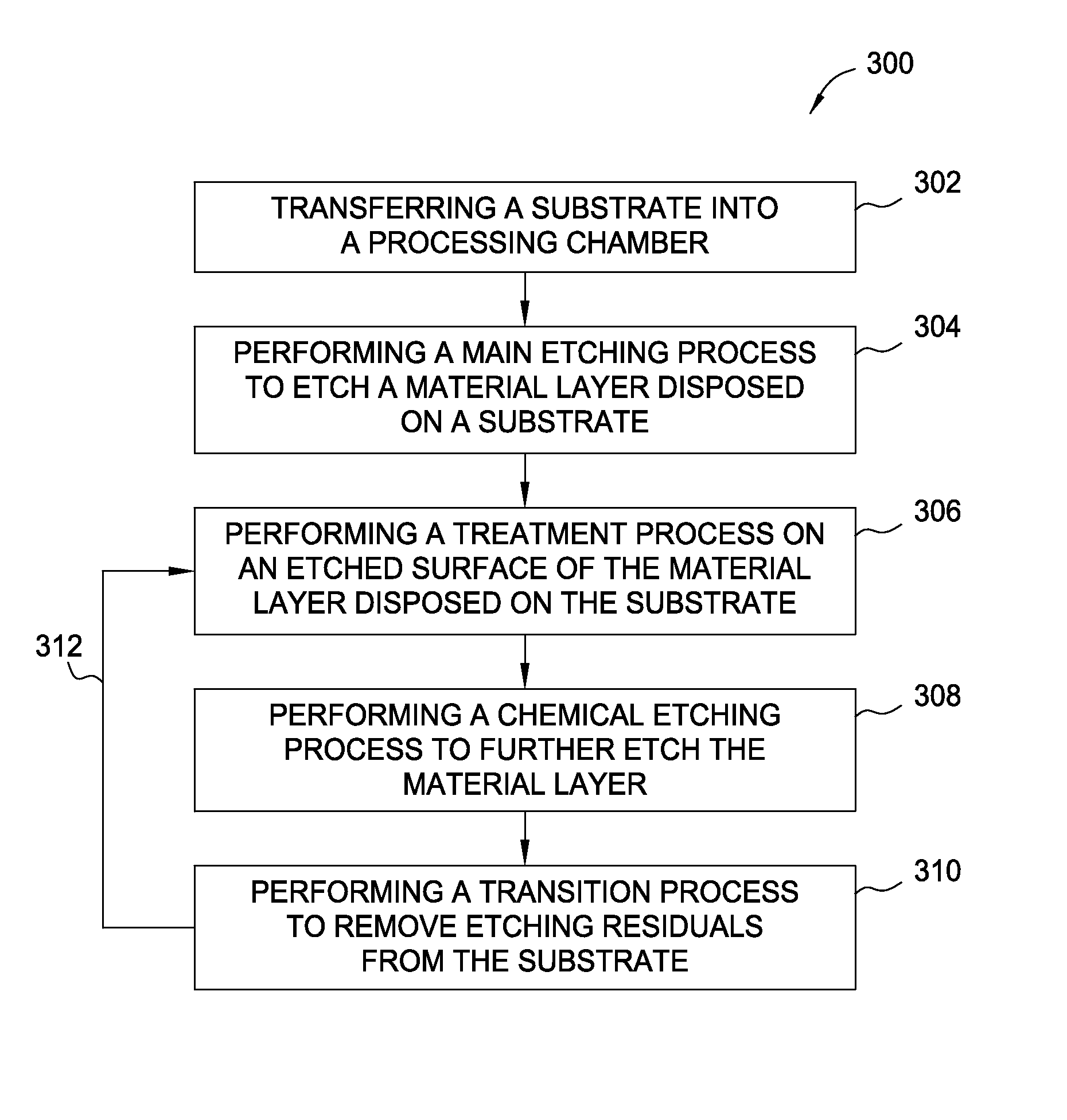
Methods for forming features in a material layer utilizing a combination of a main etching and a cyclical etching process
ActiveUS9543163B2Electric discharge tubesSemiconductor/solid-state device manufacturingEtchingCompound (substance)
Methods for etching a material layer disposed on the substrate using a combination of a main etching step and a cyclical etching process are provided. The method includes performing a main etching process in a processing chamber to an oxide layer, forming a feature with a first predetermined depth in the oxide layer, performing a treatment process on the substrate by supplying a treatment gas mixture into the processing chamber to treat the etched feature in the oxide layer, performing a chemical etching process on the substrate by supplying a chemical etching gas mixture into the processing chamber, wherein the chemical etching gas includes at least an ammonium gas and a nitrogen trifluoride, wherein the chemical etching process further etches the feature to a second predetermined depth, and performing a transition process on the etched substrate by supplying a transition gas mixture into the processing chamber.
Owner:APPLIED MATERIALS INC
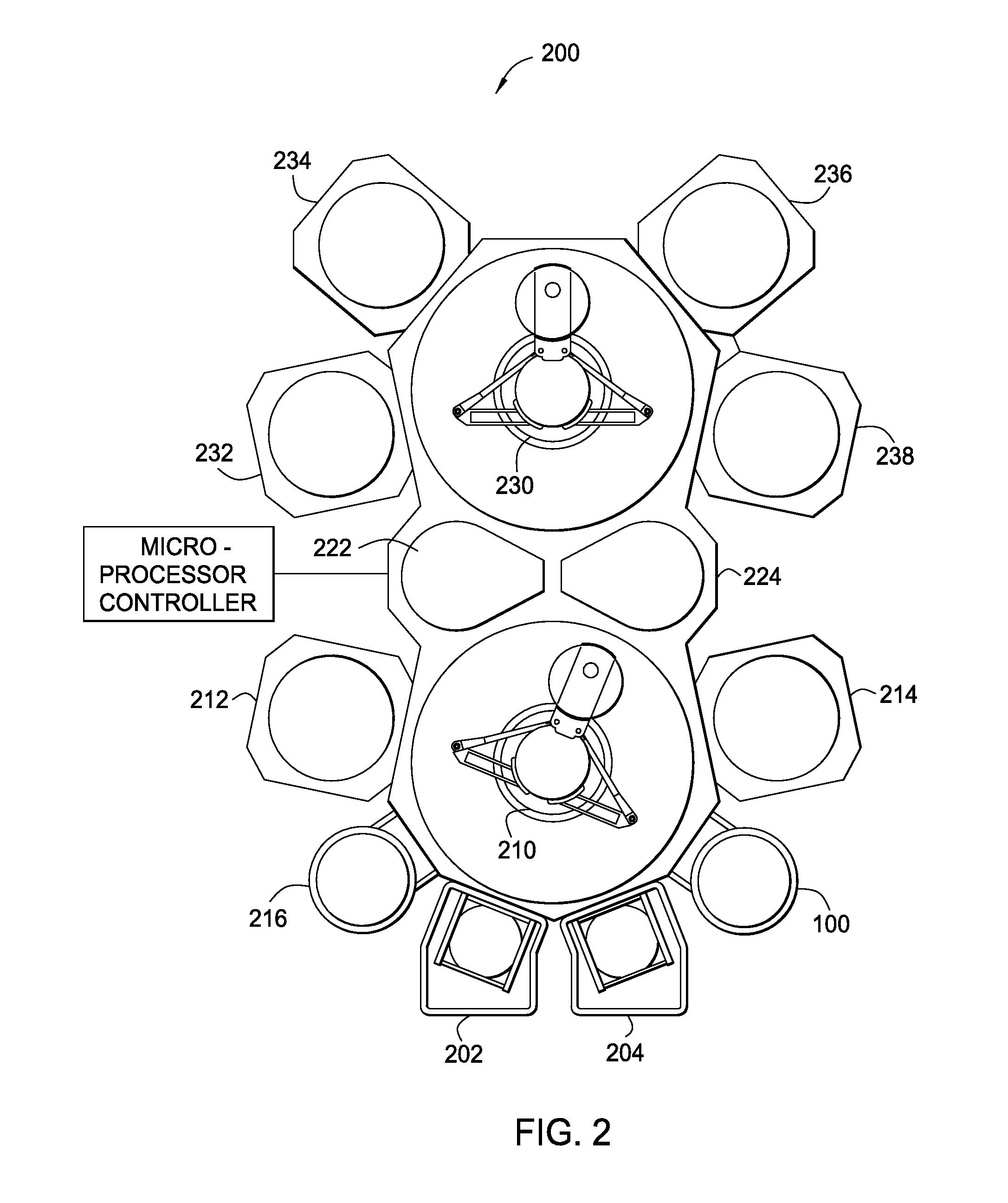
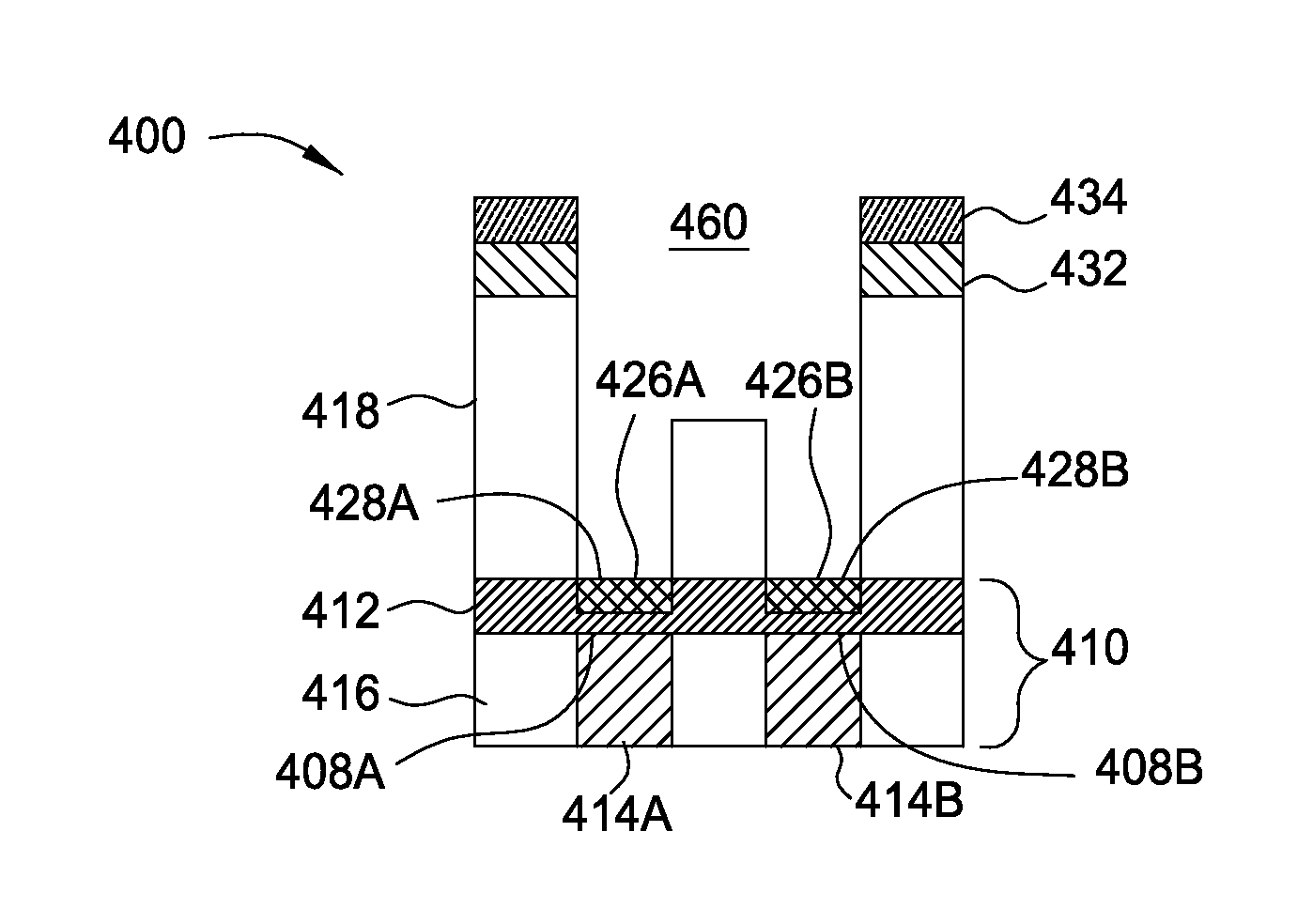
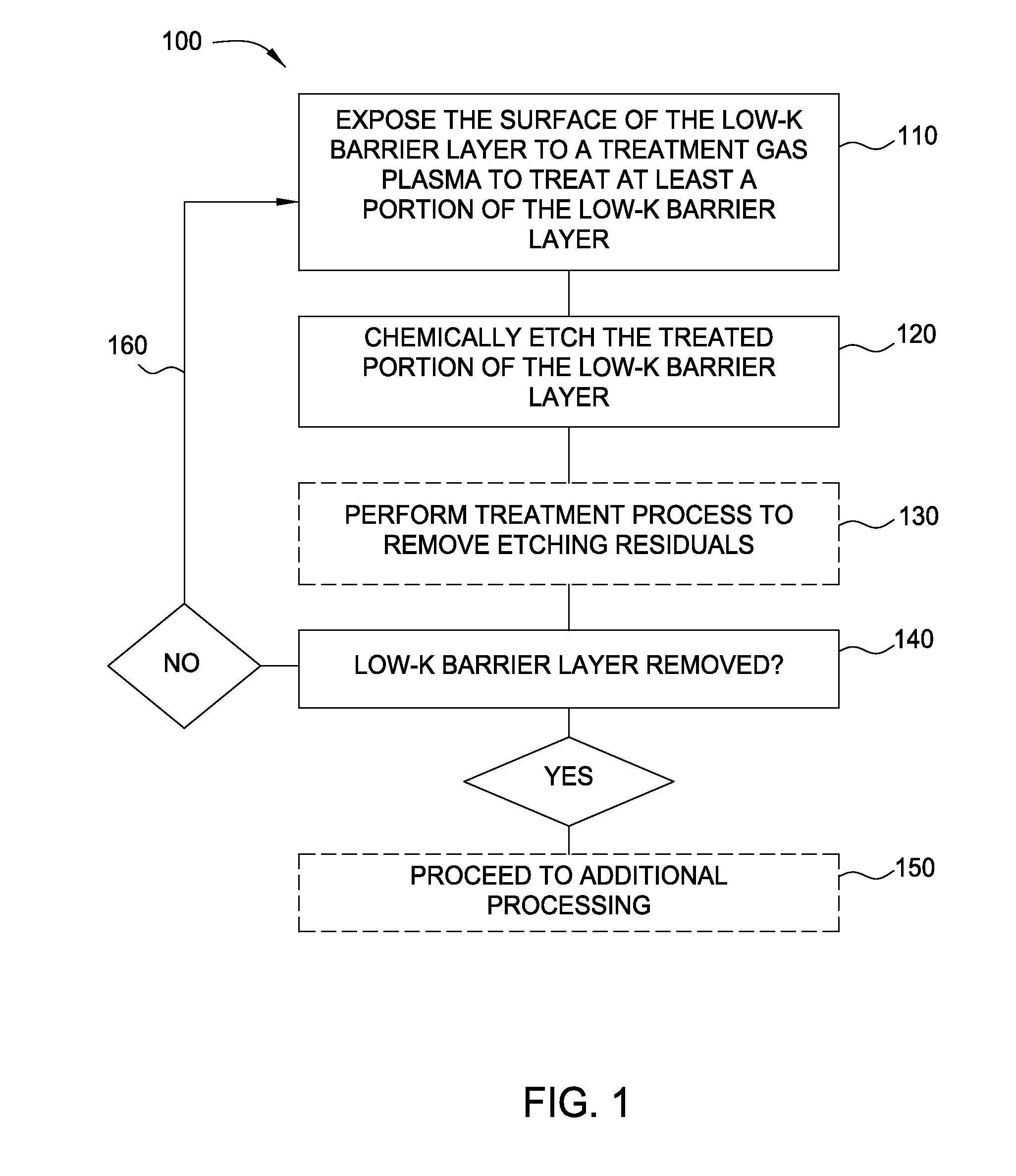
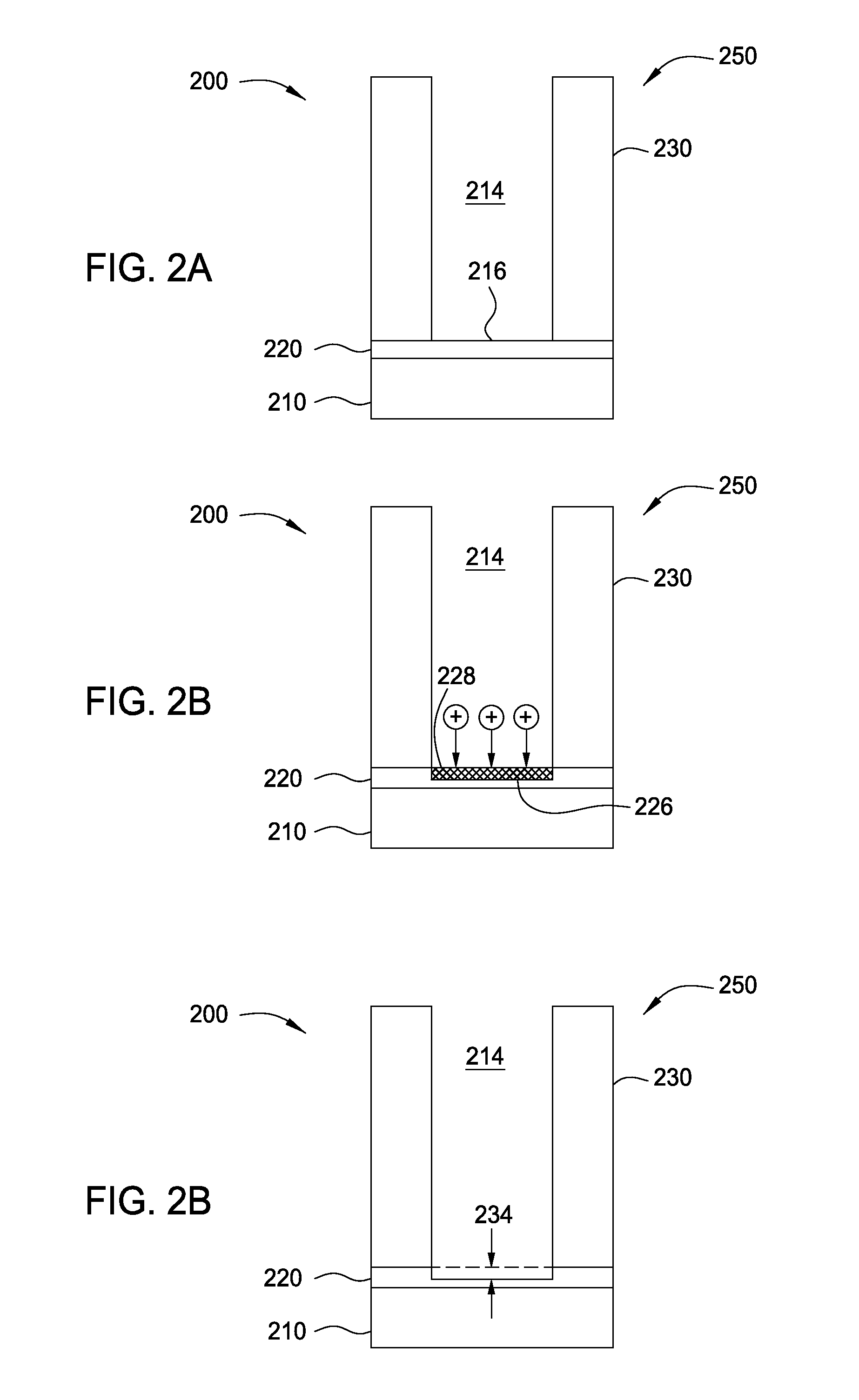
Methods for barrier layer removal
ActiveUS20150140827A1Electric discharge tubesSemiconductor/solid-state device manufacturingHydrogenSemiconductor
Implementations described herein generally relate to semiconductor manufacturing and more particularly to methods for etching a low-k dielectric barrier layer disposed on a substrate using a non-carbon based approach. In one implementation, a method for etching a barrier low-k layer is provided. The method comprises (a) exposing a surface of the low-k barrier layer to a treatment gas mixture to modify at least a portion of the low-k barrier layer and (b) chemically etching the modified portion of the low-k barrier layer by exposing the modified portion to a chemical etching gas mixture, wherein the chemical etching gas mixture includes at least an ammonium gas and a nitrogen trifluoride gas or at least a hydrogen gas and a nitrogen trifluoride gas.
Owner:APPLIED MATERIALS INC
Methods for forming features in a material layer utilizing a combination of a main etching and a cyclical etching process
ActiveUS20150056814A1Electric discharge tubesSemiconductor/solid-state device manufacturingEtchingCompound (substance)
Methods for etching a material layer disposed on the substrate using a combination of a main etching step and a cyclical etching process are provided. The method includes performing a main etching process in a processing chamber to an oxide layer, forming a feature with a first predetermined depth in the oxide layer, performing a treatment process on the substrate by supplying a treatment gas mixture into the processing chamber to treat the etched feature in the oxide layer, performing a chemical etching process on the substrate by supplying a chemical etching gas mixture into the processing chamber, wherein the chemical etching gas includes at least an ammonium gas and a nitrogen trifluoride, wherein the chemical etching process further etches the feature to a second predetermined depth, and performing a transition process on the etched substrate by supplying a transition gas mixture into the processing chamber.
Owner:APPLIED MATERIALS INC
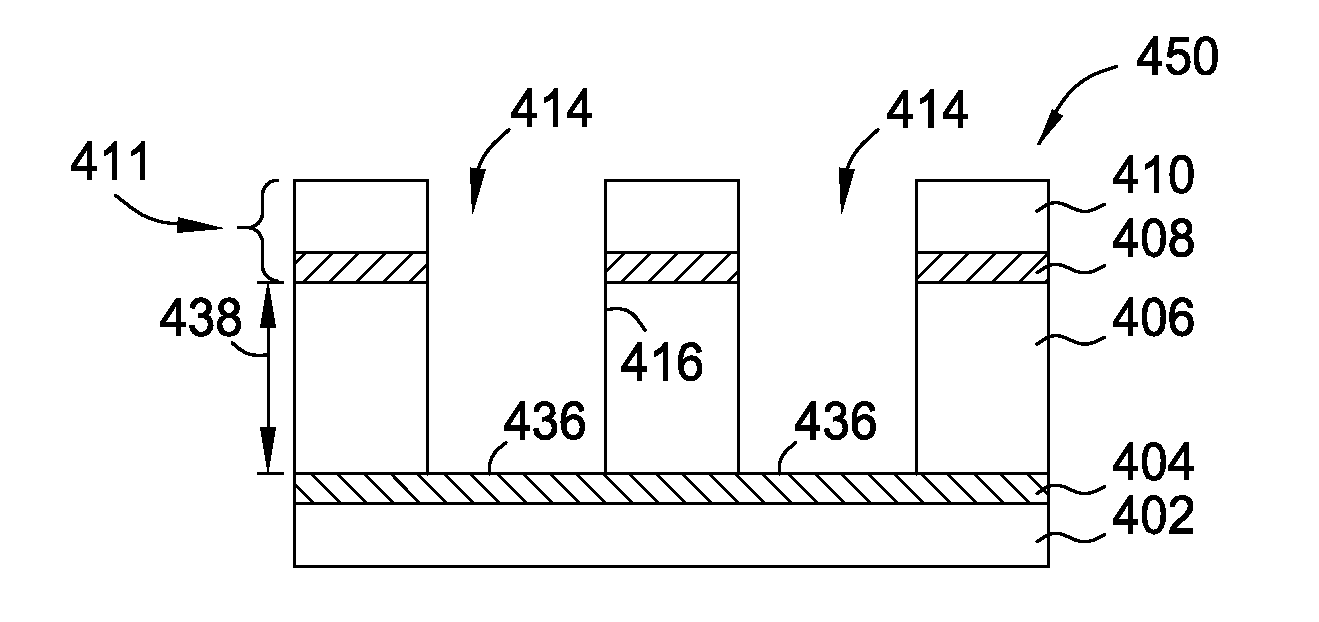
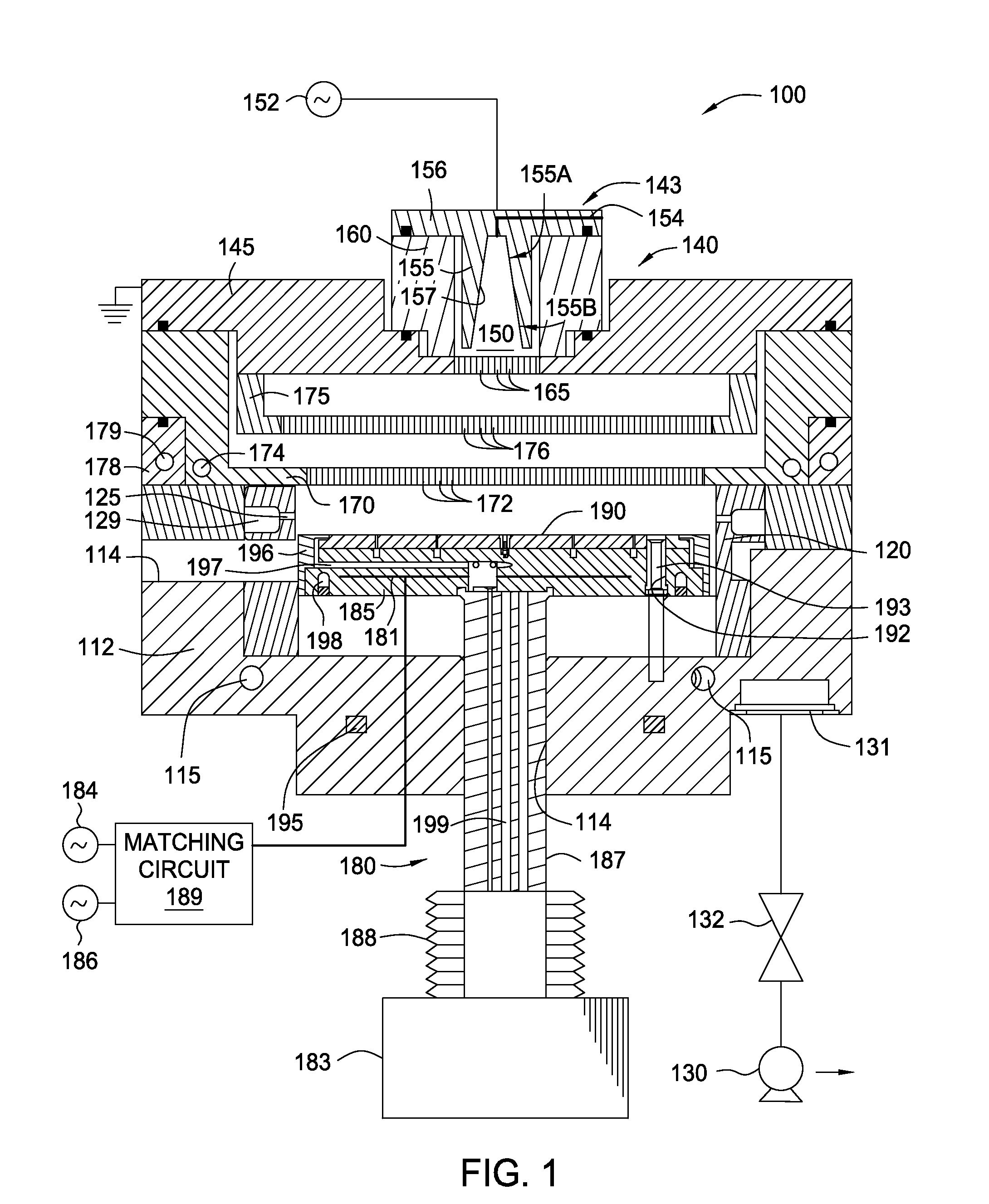
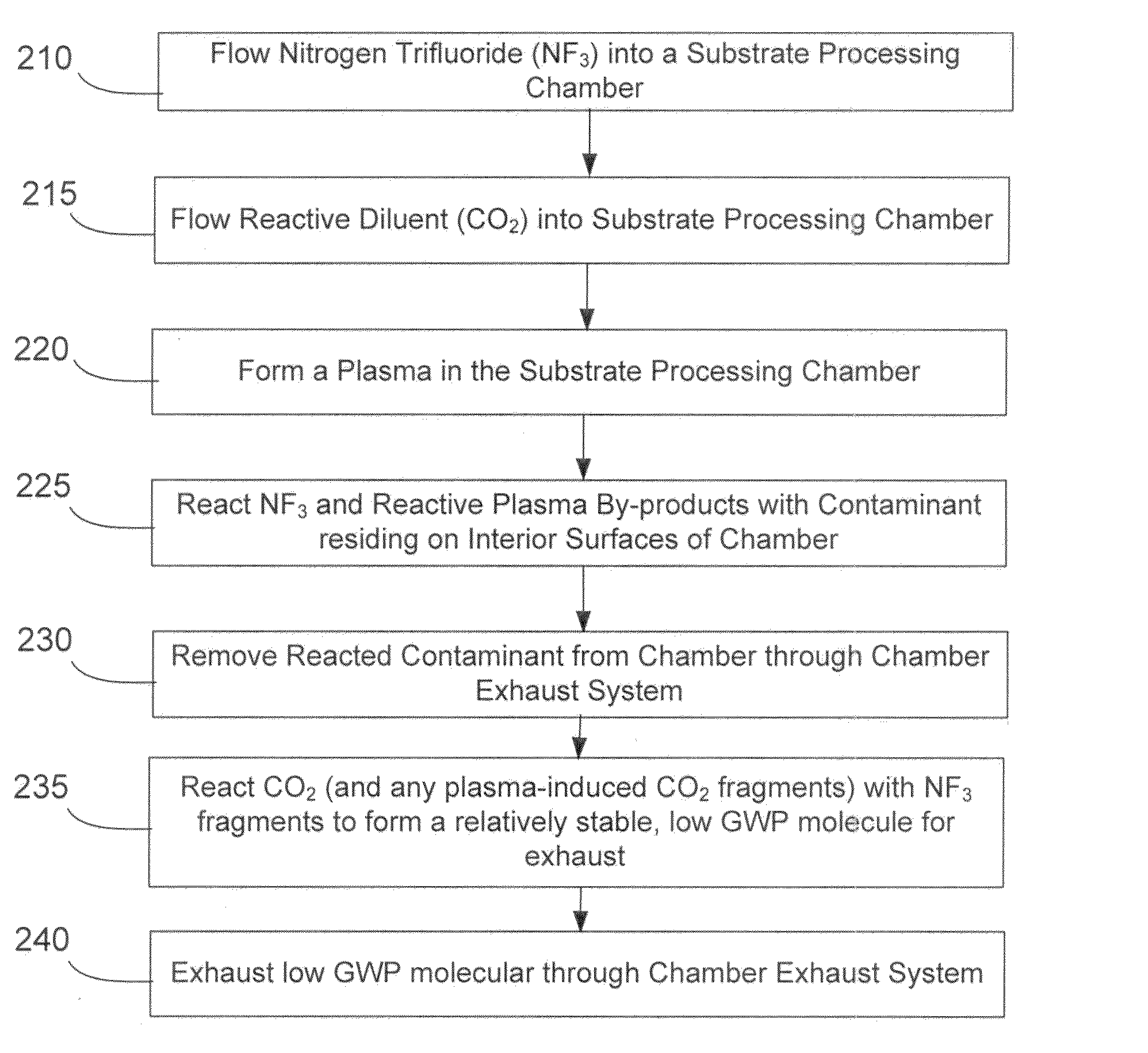
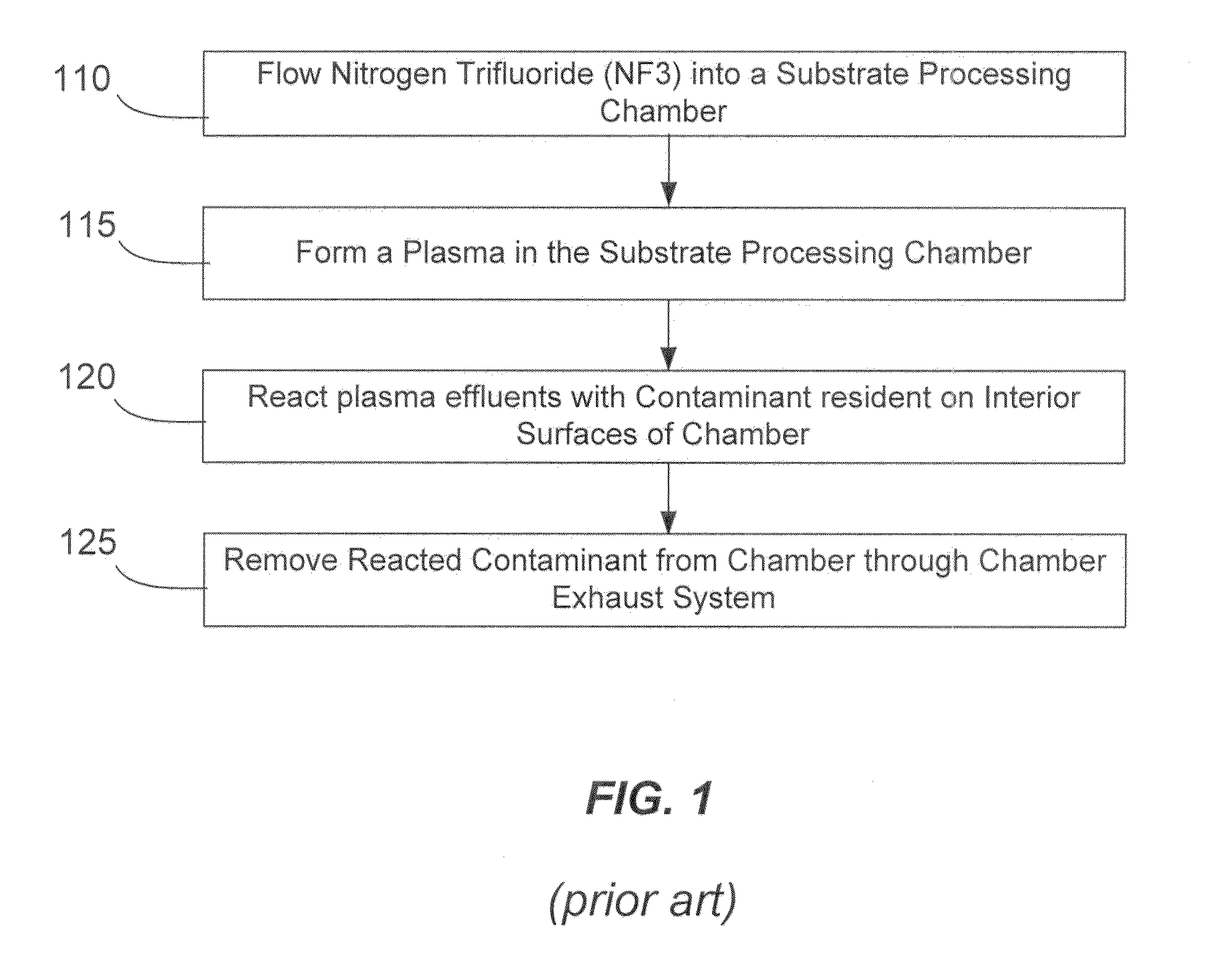
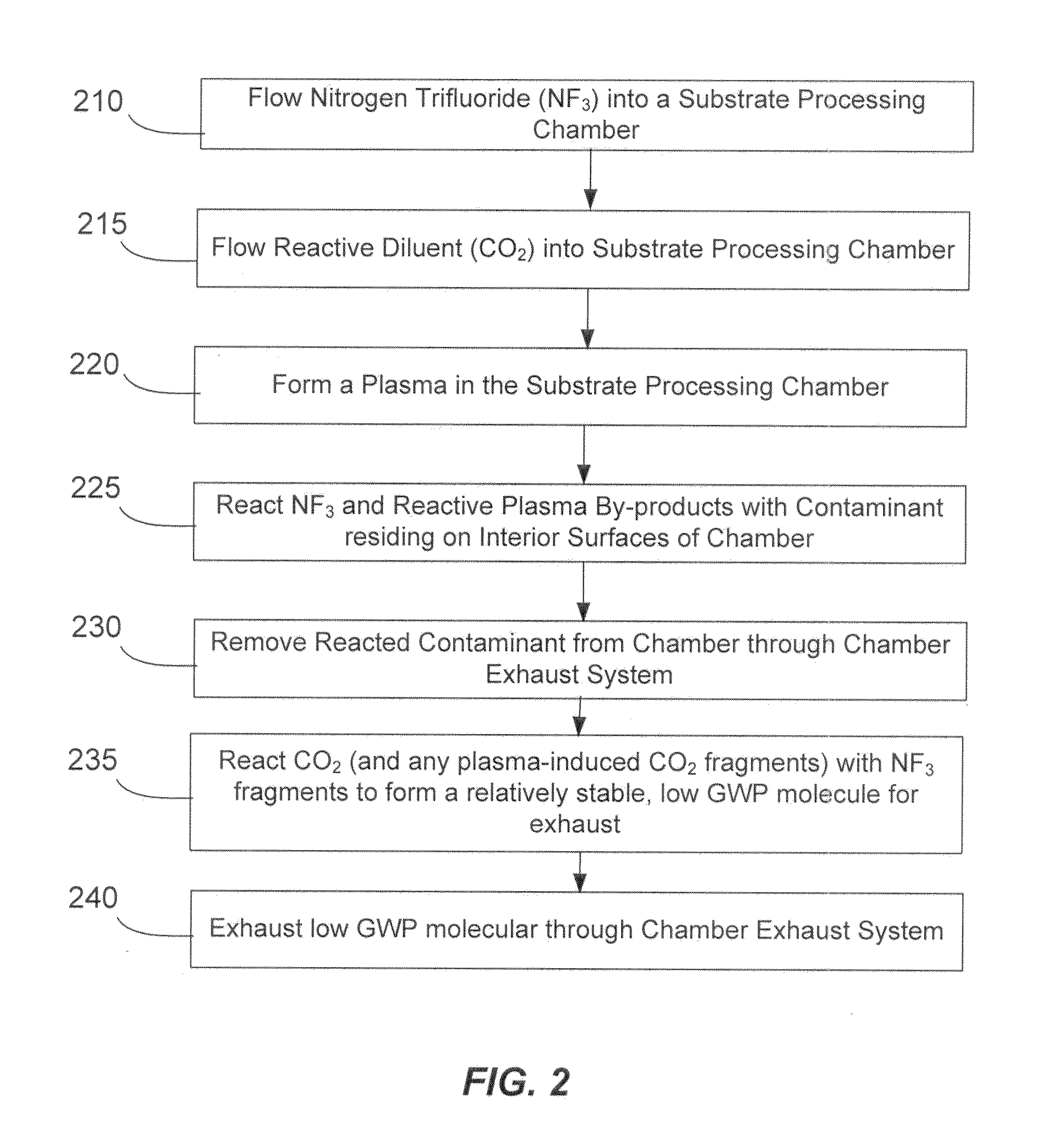
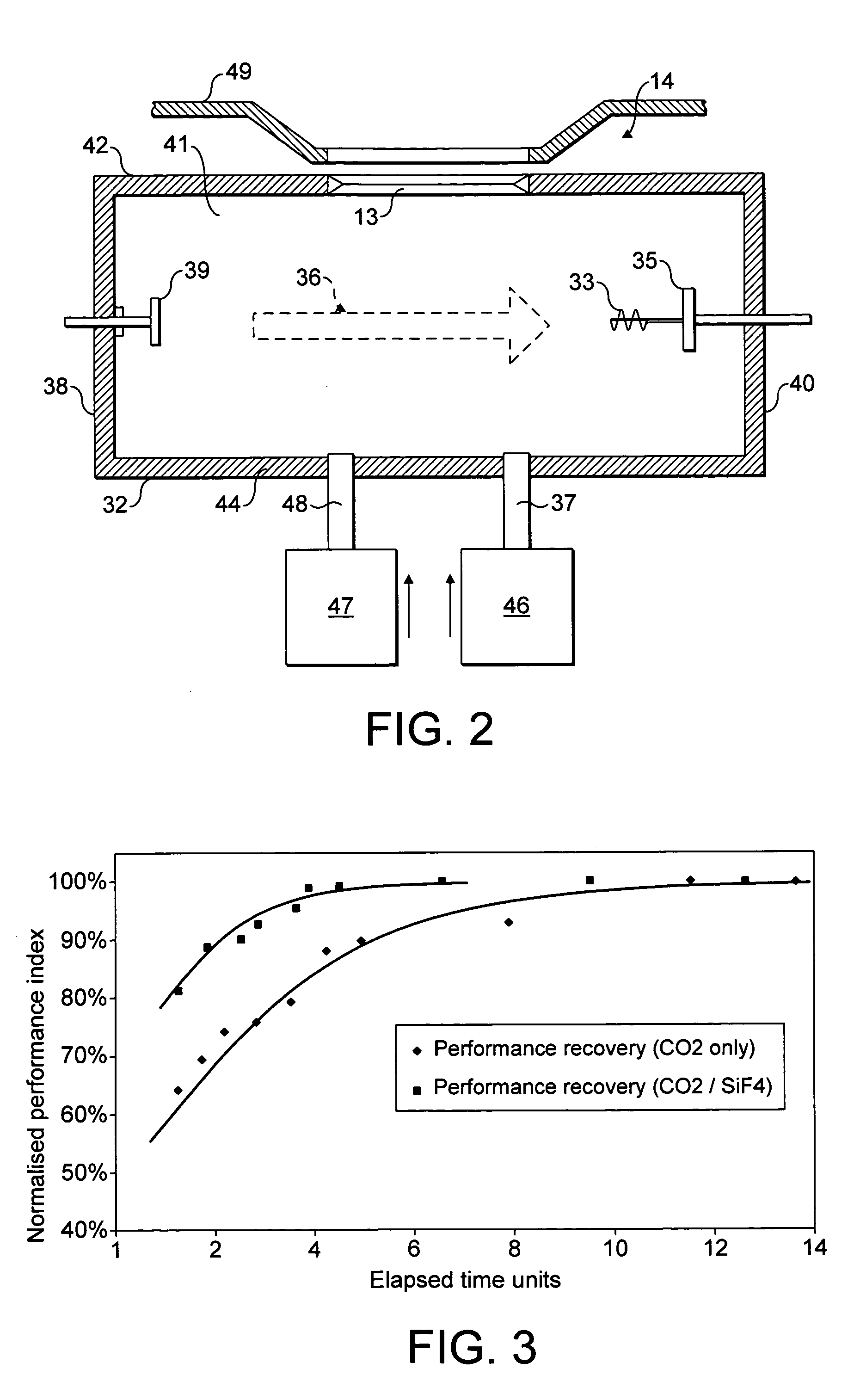
NF3 chamber clean additive
InactiveUS8623148B2Improve efficiencyReduce capacityElectric discharge tubesHollow article cleaningRemote plasmaReactive diluent
Methods of cleaning a processing chamber with nitrogen trifluoride (NF3) are described. The methods involve a concurrent introduction of nitrogen trifluoride and a reactive diluent into the chamber. The NF3 may be excited in a plasma inside the chamber or in a remote plasma region upstream from the chamber. The reactive diluent may be introduced upstream or downstream of the remote plasma such that both NF3 and the reactive diluent (and any plasma-generated effluents) are present in the chamber during cleaning. The presence of the reactive diluent enhances the chamber-cleaning effectiveness of the NF3.
Owner:MATHESON TRI GAS INC
Metal mask etching of silicon
The present disclosure provides a method for etching trenches, contact vias, or similar features to a depth of 100 mum and greater while permitting control of the etch profile (the shape of the sidewalls surrounding the etched opening). The method requires the use of a metal-comprising masking material in combination with a fluorine-comprising plasma etchant. The byproduct produced by a combination of the metal with reactive fluorine species must be essentially non-volatile under etch process conditions, and sufficiently non-corrosive to features on the substrate being etched, that the substrate remains unharmed by the etch process. Although aluminum is a preferred metal for the metal-comprising mask, other metals can be used for the masking material, so long as they produce an essentially non-volatile, non-corrosive etch byproduct under etch process conditions. By way of example, and not by way of limitation, metallic materials recommended for the mask include aluminum, cadmium, copper, chromium, gallium, indium, iron, magnesium, manganese, nickel, and combinations thereof. In particular, aluminum in combination with copper or magnesium is particularly useful, where the copper or magnesium content is less than about 8% by weight, and other constituents total less than about 2% by weight. The plasma feed gas includes at least one fluorine-containing compound such as nitrogen trifluoride (NF3), carbon tetrafluoride (CF4), and sulfur hexafluoride (SF6), by way of example and not by way of limitation. Oxygen (O2), or an oxygen-comprising compound, or hydrogen bromide (HBr), or a combination thereof may be added to the plasma feed gases to help provide a protective layer over etched sidewalls, assisting in profile control of the etched feature.
Owner:APPLIED MATERIALS INC
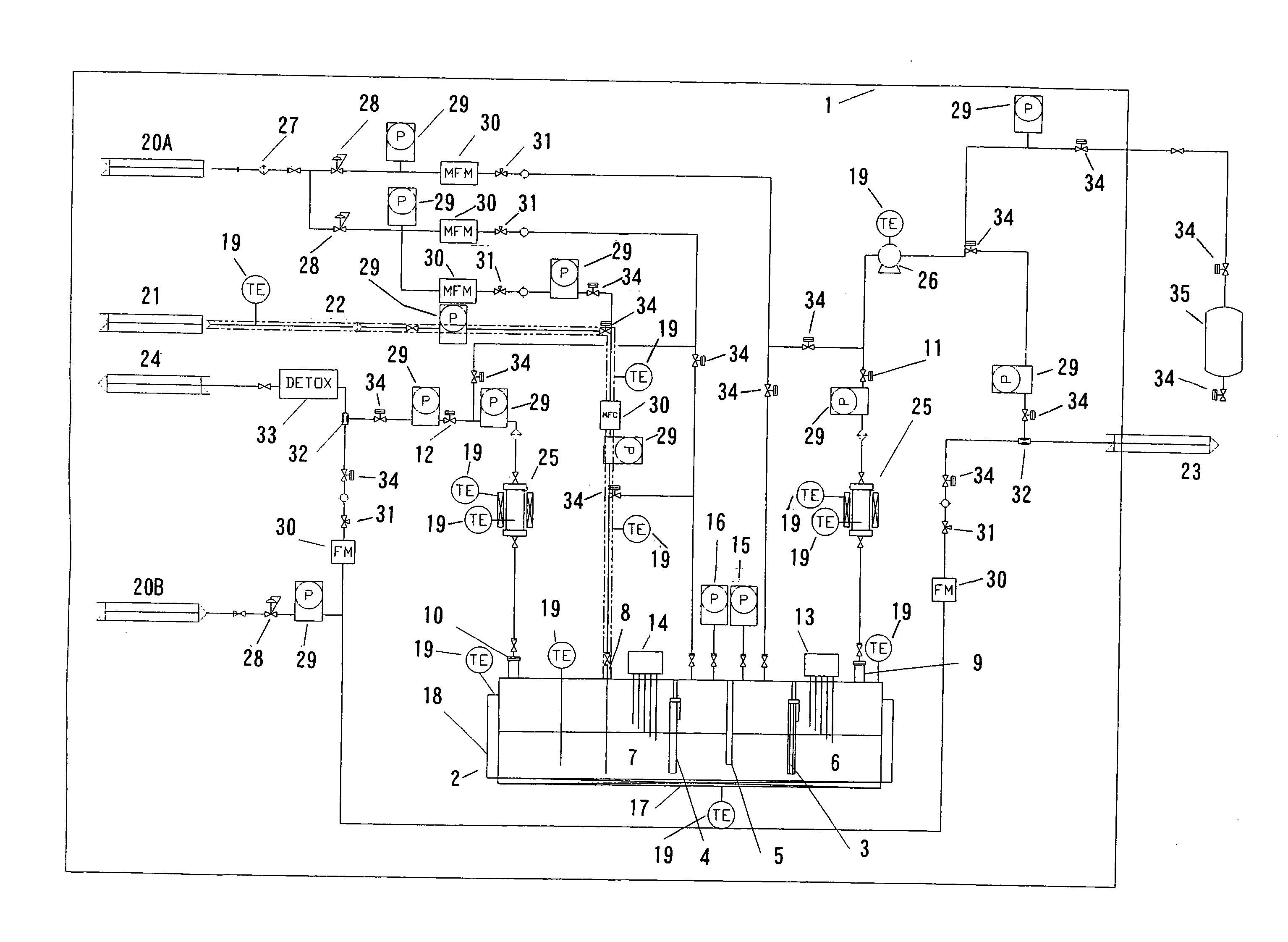
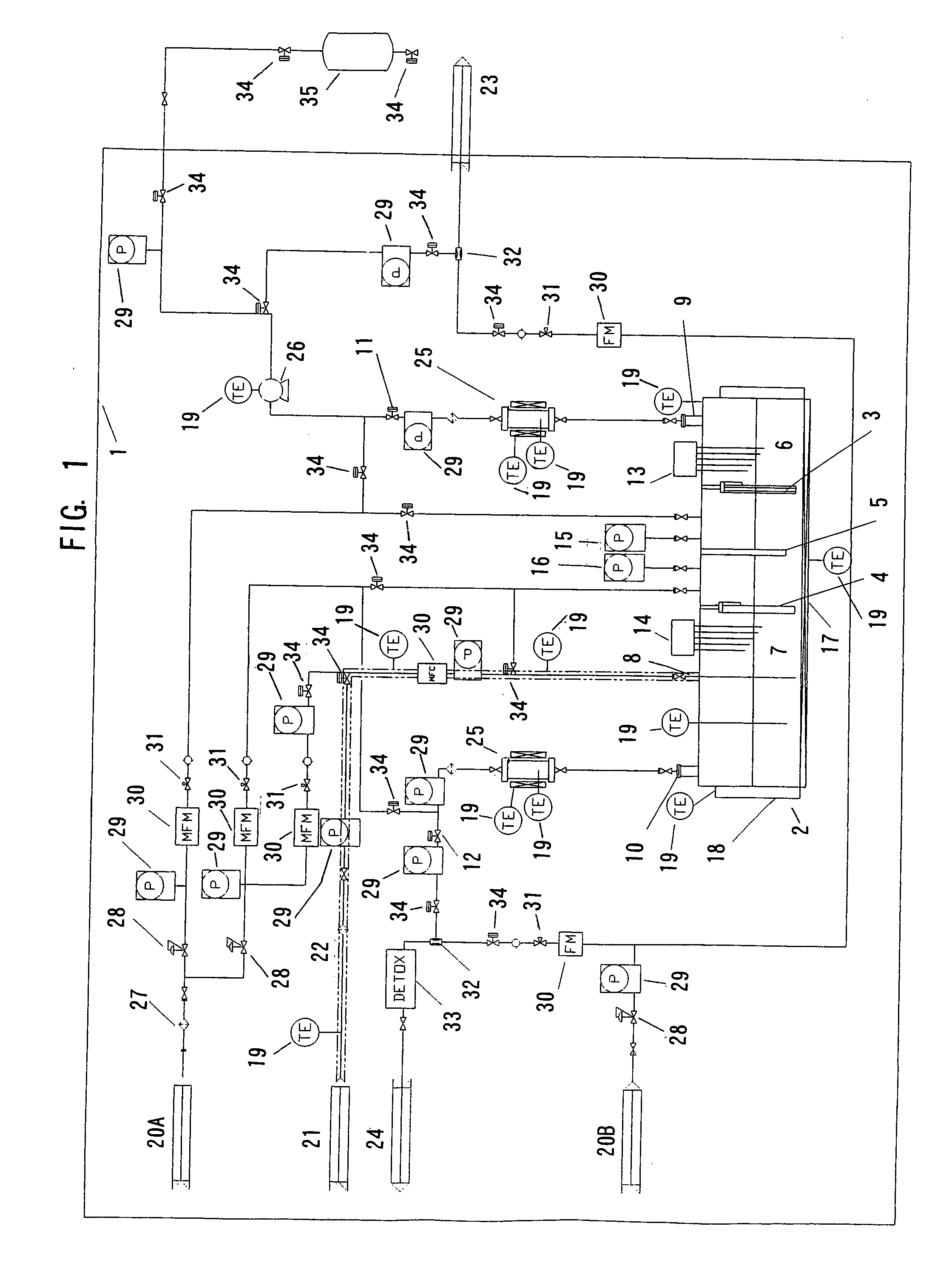
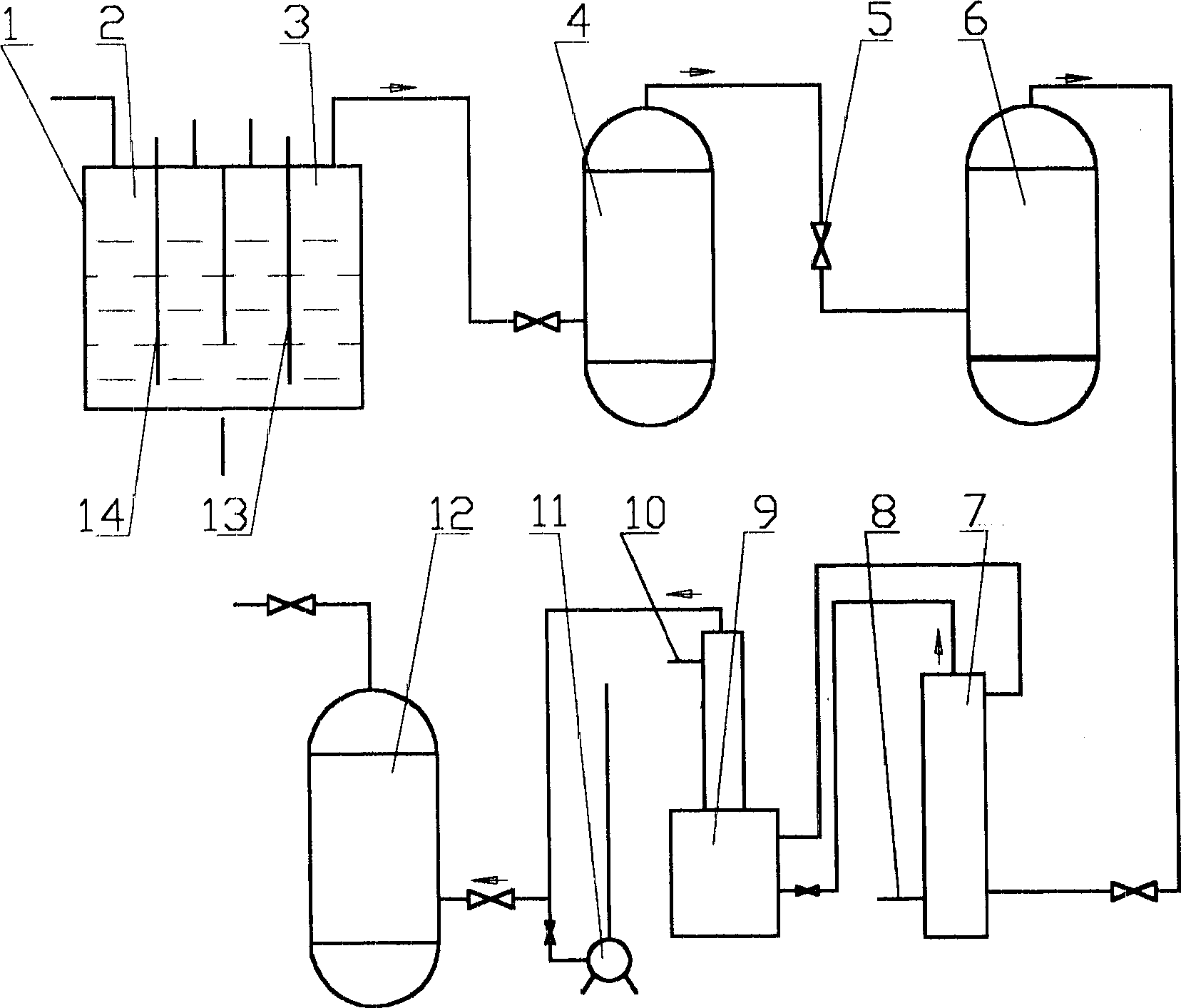
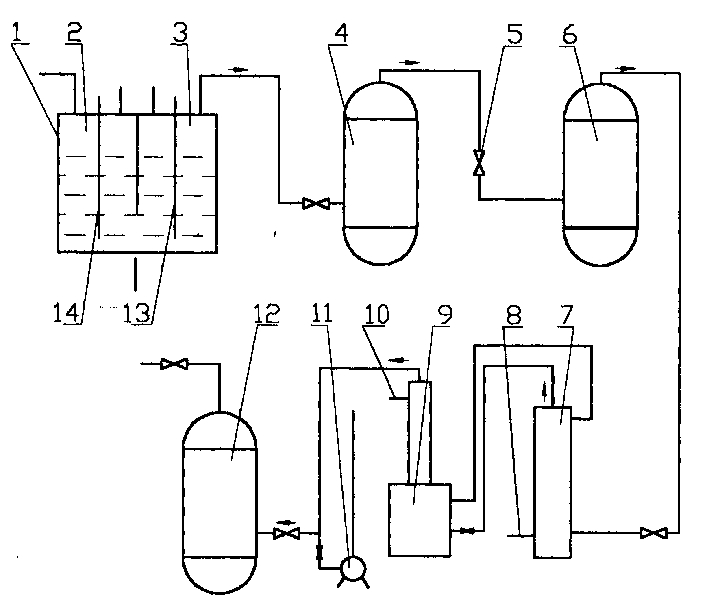
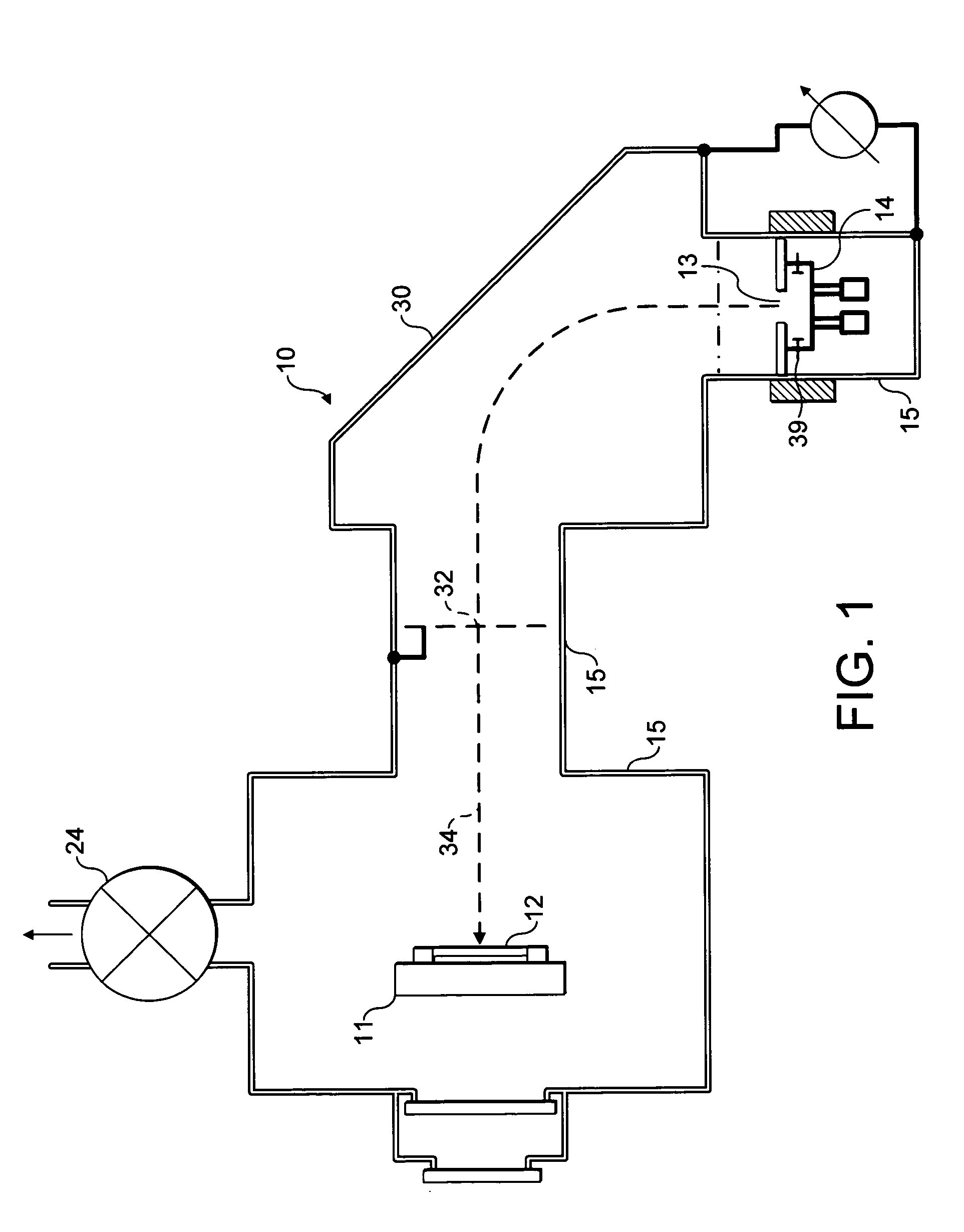
Nitrogen trifluoride production apparatus
InactiveUS6010605AHigh purityReduce impurityElectrolysis componentsNitrogen trifluorideHydrogen fluorideBoron trifluoride
Apparatus is disclosed for the production of nitrogen trifluoride (NF3), starting with an anhydrous molten flux including ammonia (NH3), a metal fluoride (MF), and hydrogen fluoride (HF). The apparatus includes an electrolyzer, an ammonia solubilizer, a hydrogen fluoride solubilizer, a nitrogen trifluoride reactor, two compressors, two pumps, three condensers a gas recycle loop, and, two flux loops of the same component ternary flux, but each loop with different concentration.
Owner:FLORIDA SCI LAB
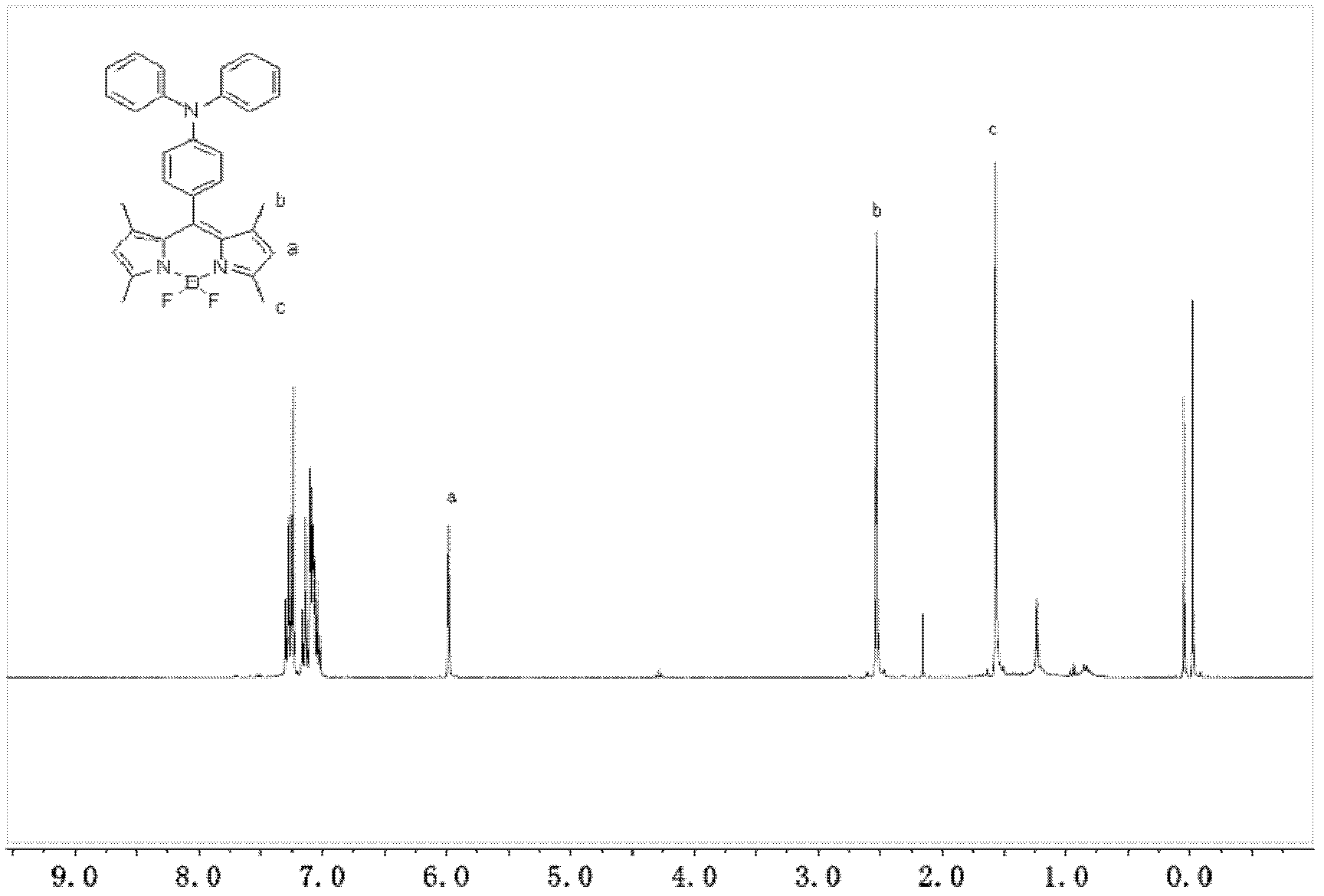
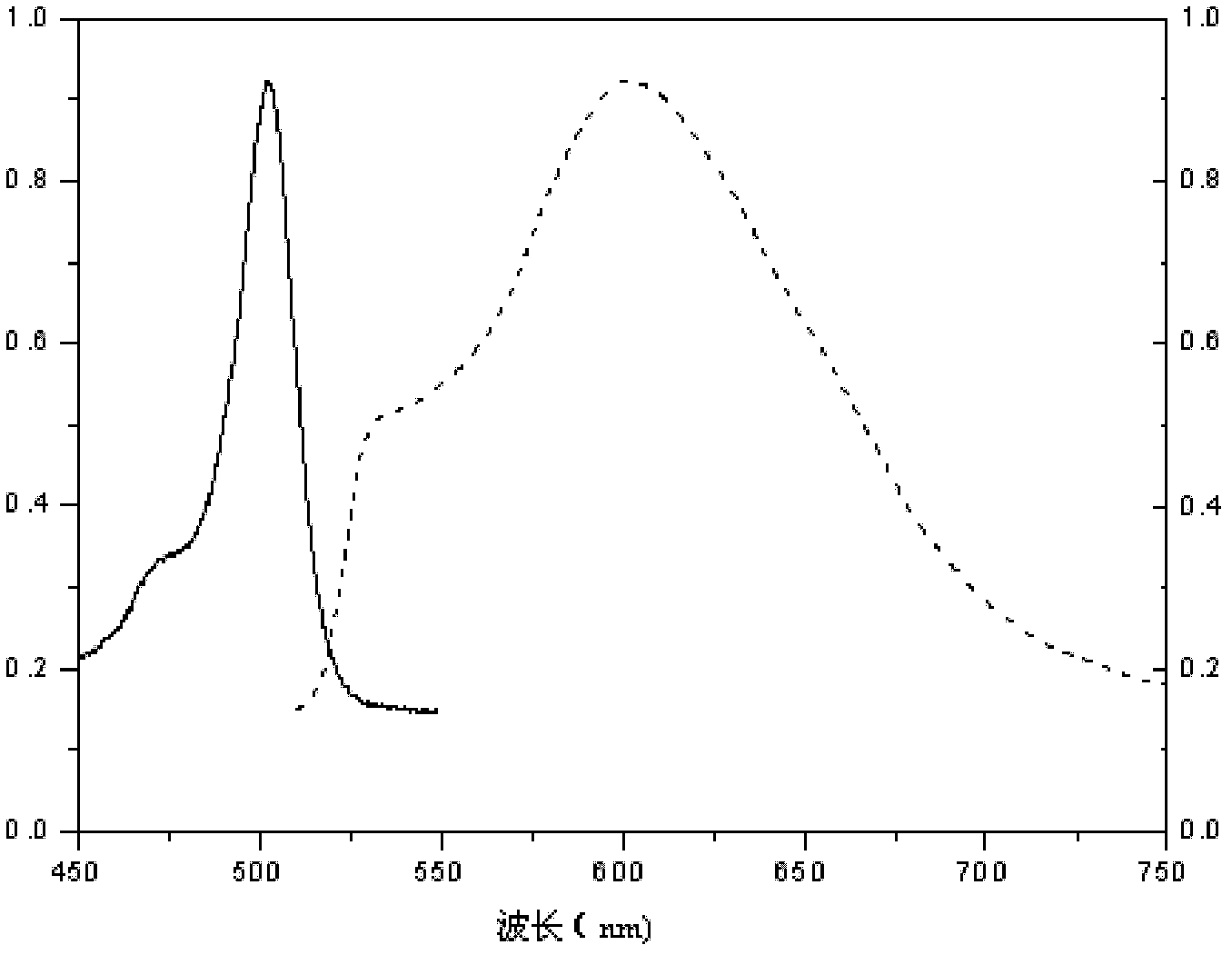
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method of etching tungsten or tungsten nitride electrode gates in semiconductor structures
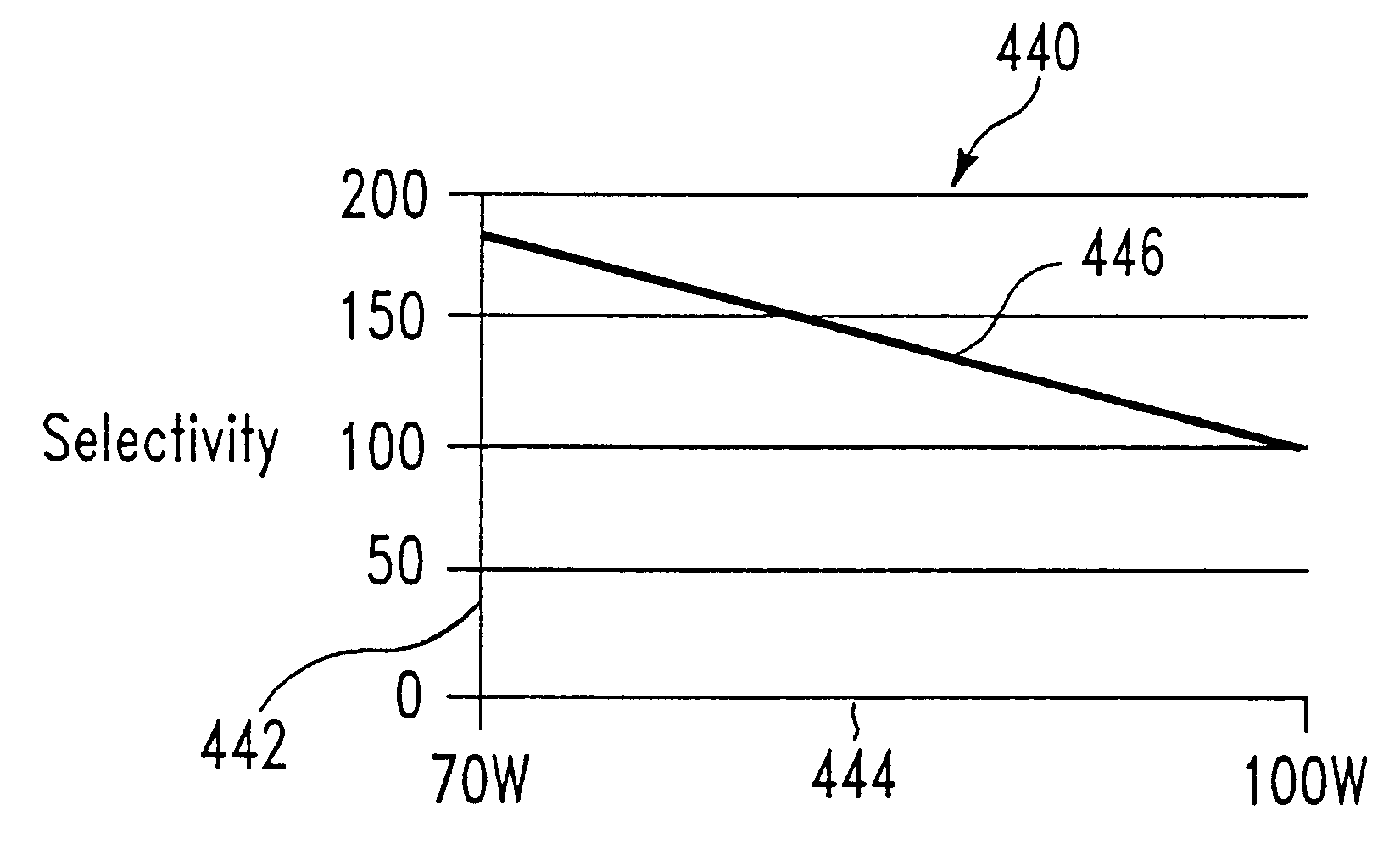
InactiveUS6440870B1Decorative surface effectsSemiconductor/solid-state device detailsEtchingSulfur hexafluoride
The present invention relates to a method of etching tungsten or tungsten nitride in semiconductor structures, and particularly to the etching of gate electrodes which require precise control over the etching process. We have discovered a method of etching tungsten or tungsten nitride which permits precise etch profile control while providing excellent selectivity, of at least 175:1, for example, in favor of etching tungsten or tungsten nitride rather than an adjacent oxide layer. Typically, the oxide is selected from silicon oxide, silicon oxynitride, tantalum pentoxide, zirconium oxide, and combinations thereof. The method appears to be applicable to tungsten or tungsten nitride, whether deposited by physical vapor deposition (PVD) or chemical vapor deposition (CVD). In particular, an initial etch chemistry, used during the majority of the tungsten or tungsten nitride etching process (the main etch), employs the use of a plasma source gas where the chemically functional etchant species are generated from a combination of sulfur hexafluoride (SF6) and nitrogen (N2), or in the alternative, from a combination of nitrogen trifluoride (NF3), chlorine (Cl2), and carbon tetrafluoride (CF4). Toward the end of the main etching process, a second chemistry is used in which the chemically functional etchant species are generated from Cl2 and O2. This final portion of the etch process may be referred to as an "overetch" process, since etching is carried out to at least the surface underlying the tungsten or tungsten nitride. However, this second etch chemistry may optionally be divided into two steps, where the plasma source gas oxygen content and plasma source power are increased in the second step.
Owner:APPLIED MATERIALS INC
Electrolytic apparatus for producing fluorine or nitrogen trifluoride
InactiveUS20070215460A1Avoid it happening againSuppression amountCellsMachining electric circuitsHigh current densityHydrogen fluoride
It is a task of the present invention to provide an electrolytic apparatus for producing fluorine or nitrogen trifluoride by electrolyzing a hydrogen fluoride-containing molten salt, the electrolytic apparatus being advantageous in that the electrolysis can be performed without the occurrence of the anode effect even at a high current density and without the occurrence of an anodic dissolution. In the present invention, this task has been accomplished by an electrolytic apparatus for producing fluorine or nitrogen trifluoride by electrolyzing a hydrogen fluoride-containing molten salt at an applied current density of from 1 to 1,000 A / dm2, the electrolytic apparatus using a conductive diamond-coated electrode as an anode.
Owner:TOYO TANSO KK
Method of photoresist strip for plasma doping process of semiconductor manufacturing
ActiveUS20070243700A1Sure easySemiconductor/solid-state device manufacturingSemiconductor devicesDopantHexafluoroethane
A method of forming an intermediate semiconductor device is disclosed that comprises providing a semiconductor substrate, forming a photoresist layer on the semiconductor substrate, implanting a dopant into the semiconductor substrate, and removing a dopant-containing layer from the photoresist layer. The dopant-containing layer includes dopant residuals and a carbon-rich crust and may be formed during implantation. The dopant-containing layer may be removed from the photoresist layer by exposing the dopant-containing layer to a water rinse, a chlorinated plasma or to a fluorinated plasma. The water rinse may include deionized water that is maintained at a temperature that ranges from approximately 25° C. to approximately 80° C. The fluorinated plasma may be formed from a gaseous precursor selected from the group consisting of nitrogen trifluoride, carbon tetrafluoride, trifluoromethane, hexafluoroethane, sulfur hexafluoride, and mixtures thereof. A method of forming an ultrashallow junction is also disclosed.
Owner:MICRON TECH INC
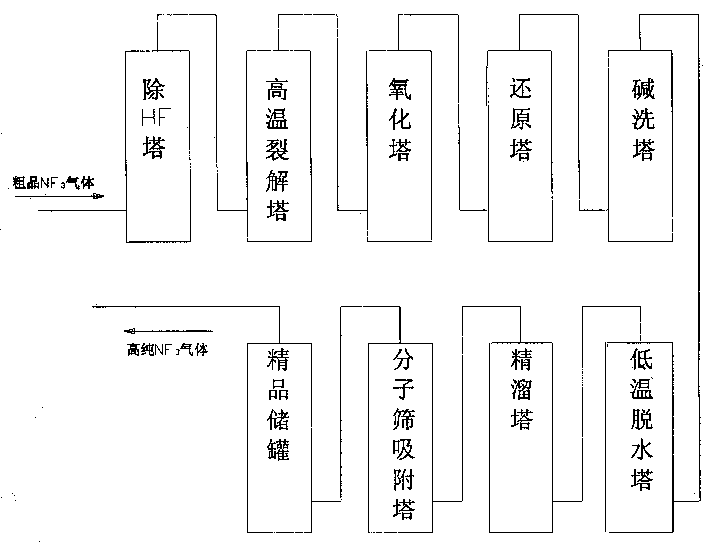
Technological method and equipment for preparing nitrogen trifluoride gas
InactiveCN1450202AHigh purityReduce the difficulty of purificationElectrolysis componentsCooling towerBoiling point
The present invention relates to a process for preparing NF3 gas and its equipment. It is characterized by that the anodic electrolytic gas in the electrolytic cell is fed into a pressure balancing tank by means of pipe, then fed into a low pressure tank via regulating valve, and said gas is fed into a low-temp. cooling tower by means of pipe to remove high-boiling point impurities of NH4F and HFfrom said anodic electrolytic gas to obtain NF3 gas, then the NF3 gas is fed into an ultralow temp. cold trap to make liquification and collection, and remove low-boiling point impurities or N2 and O2 from it by adopting the measure of evacuating cold trap, after the NF3 gas is collected and liquified in the ultralow-temp. cold trap, heating the cold trap and distilling said NF3 gas and pressing it into crude NF3 gas storage tank, the material of anode in the electrolytic cell is high-purity nickel.
Owner:PERIC SPECIAL GASES CO LTD
Method of recycling fluorine using an adsorption purification process
InactiveUS6955707B2Final product manufactureSemiconductor/solid-state device manufacturingSorbentImpurity
A method and apparatus is disclosed for producing fluorine by providing a contained fluorine precursor source located proximate to or remotely from an adsorbent bed, optionally in a replaceable unit that may be a replaceable module comprising both the fluorine source and the adsorbent bed. Fluorine derived preferably from a nitrogen trifluoride source and used to remove deposited silicon-containing impurities in reaction chambers is reclaimed from an adsorbent bed, and made available to the reaction chamber as a supplemental fluorine source to reduce the total required amount of nitrogen trifluoride source gas. The separation column adsorbent is regenerated in cyclical intervals using a reverse flow of inert gas.
Owner:BOC GRP INC
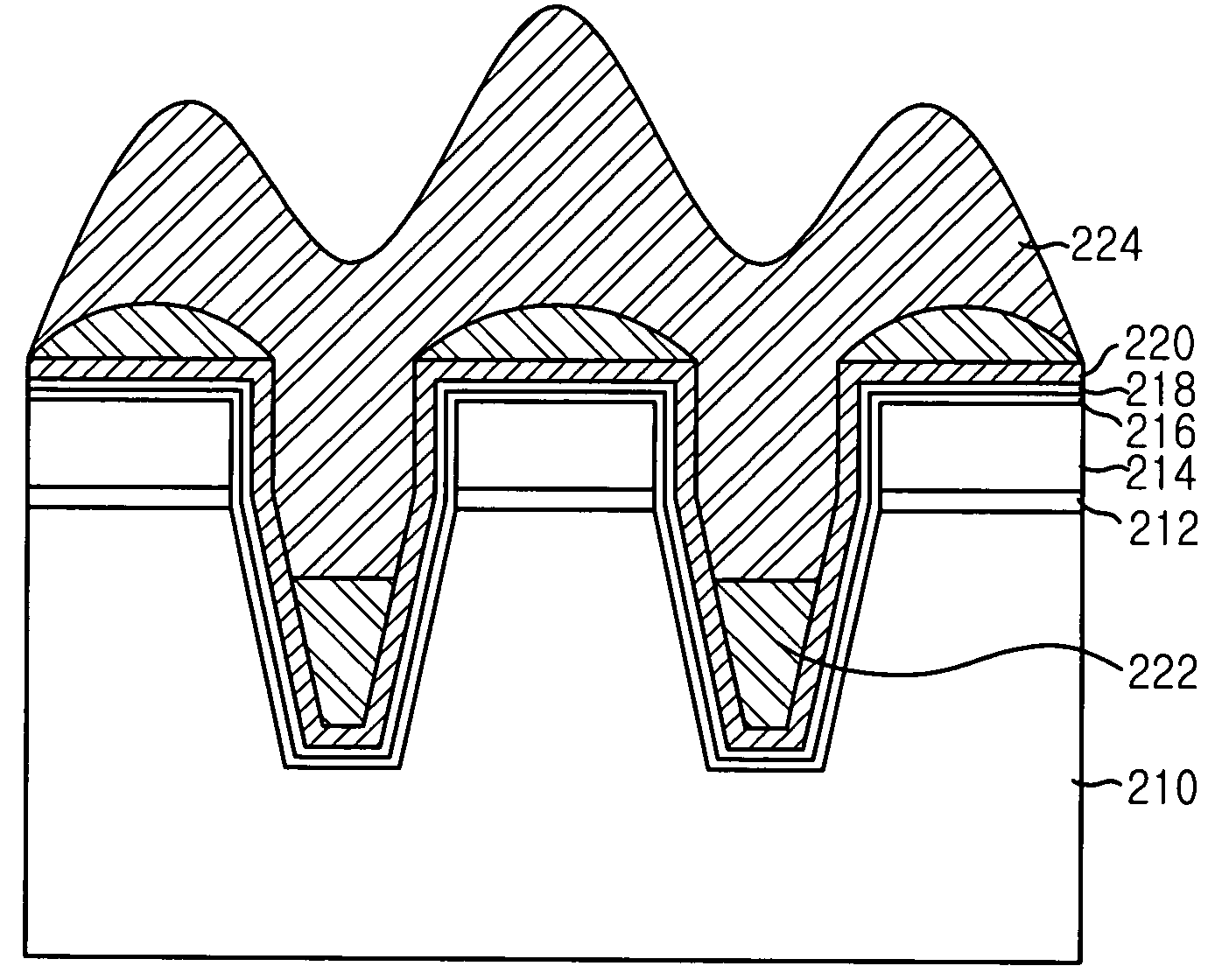
Method for manufacturing shallow trench isolation in semiconductor device
InactiveUS6998326B2Enhanced gap-fill propertyImprove propertiesSemiconductor/solid-state device manufacturingHigh densityDevice material
The method for manufacturing a shallow trench isolation (STI) in a semiconductor device with an enhanced gap-fill property and without a detrimental effect of fluorine by introducing a two-stage thermal process. The method includes steps of: preparing a semiconductor substrate obtained by a predetermined process on which a pad oxide and a pad nitride are formed on predetermined locations thereof; forming a trench structure in the semiconductor substrate; forming a hydrogen (H2)-based high density plasma (HDP) oxide layer over a first resultant structure; forming a nitrogen trifluoride (NF3)-based HDP oxide layer into the trench structure with a predetermined depth; carrying out a two-stage thermal process for removing fluorine in the NF3-based HDP oxide layer; and forming a helium (He)-based HDP oxide layer over a second resultant structure.
Owner:SK HYNIX INC
Method of photoresist strip for plasma doping process of semiconductor manufacturing
ActiveUS7737010B2Semiconductor/solid-state device manufacturingSemiconductor devicesHexafluoroethaneDopant
A method of forming an intermediate semiconductor device is disclosed that comprises providing a semiconductor substrate, forming a photoresist layer on the semiconductor substrate, implanting a dopant into the semiconductor substrate, and removing a dopant-containing layer from the photoresist layer. The dopant-containing layer includes dopant residuals and a carbon-rich crust and may be formed during implantation. The dopant-containing layer may be removed from the photoresist layer by exposing the dopant-containing layer to a water rinse, a chlorinated plasma or to a fluorinated plasma. The water rinse may include deionized water that is maintained at a temperature that ranges from approximately 25° C. to approximately 80° C. The fluorinated plasma may be formed from a gaseous precursor selected from the group consisting of nitrogen trifluoride, carbon tetrafluoride, trifluoromethane, hexafluoroethane, sulfur hexafluoride, and mixtures thereof. A method of forming an ultrashallow junction is also disclosed.
Owner:MICRON TECH INC
Ion sources
ActiveUS20050211923A1Reduce formationImprove efficiencySemiconductor/solid-state device manufacturingMaterial analysis by optical meansChemical reactionPhysical chemistry
The invention relates to methods of controlling the effect of ions of an ionisable source gas that can react with interior surfaces of an arc chamber, by introducing ions of a displacement gas into the arc chamber, where the displacement gas ions are more chemically reactive with the material of the interior surfaces than the ions of the source gas. The source gas ions may typically be oxygen ions and the displacement gas ions are then typically fluorine ions where the interior surfaces comprise tungsten. The fluorine ions may, by way of example, be sourced from fluorine, silicon tetrafluoride or nitrogen trifluoride.
Owner:APPLIED MATERIALS INC
Fine preparation process for high-purity nitrogen trifluoride gas
InactiveCN1450203AHigh puritySimple processElectrolysis componentsFluoride preparationElectrolytic agentMolecular sieve
The present invention relates to a method for refining high-purity NF gas by using crude product NF3 gas as raw material gas produced by electrolysis using NF4F-xHF as electrolyte system, and said method includes the following steps: A. removing impurities of H2O and HF from raw material gas in HF-removing tower; B. removing NxFy and FxOy from raw material gas in high-temp. cracking tower; C. removing N2O impurity from raw material gas in oxidation tower; D. removing oxidation substances produced in above-mentioned high-temp. cracking and oxidation steps of B and C in reduction tower; E. removing acidic substances from raw material gas in alkali-washing tower; F. removing water from raw material gas in low-temp. dewatering tower; G rectifying in rectifying tower.
Owner:PERIC SPECIAL GASES CO LTD
Nitrogen sources for molecular beam epitaxy
InactiveUS7255746B2Polycrystalline material growthSemiconductor/solid-state device manufacturingDimethylhydrazineNitrogen source
MBE nitrogen sources of dimethylhydrazine, tertiarybutlyhydrazine, nitrogentrifloride, and NHx radicals. Those nitrogen sources are beneficial in forming nitrogen-containing materials on crystalline subtrates using MBE. Semiconductor lasers in general, and VCSEL in particular, that have nitrogen-containing layers can be formed using such nitrogen sources.
Owner:II VI DELAWARE INC
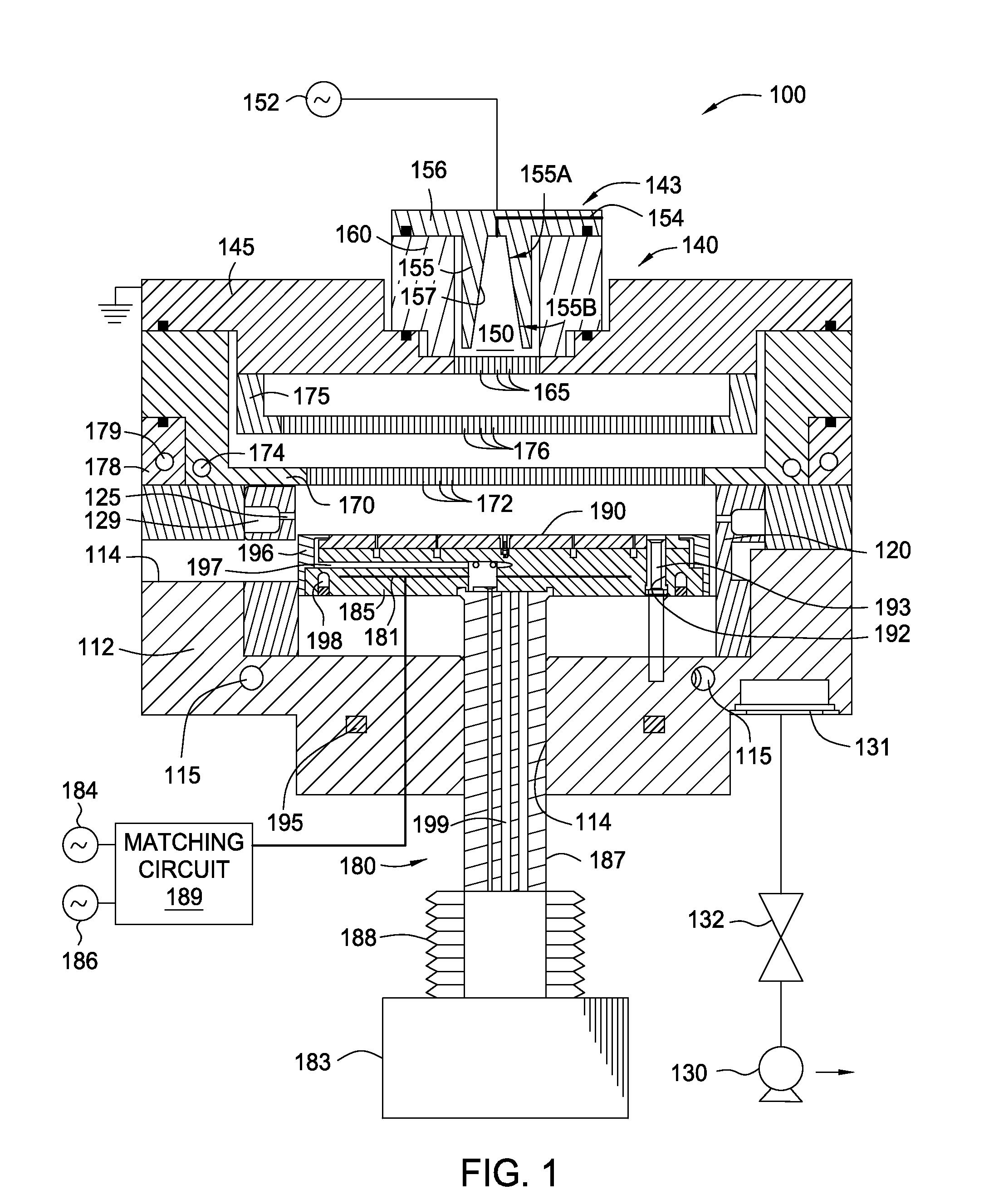
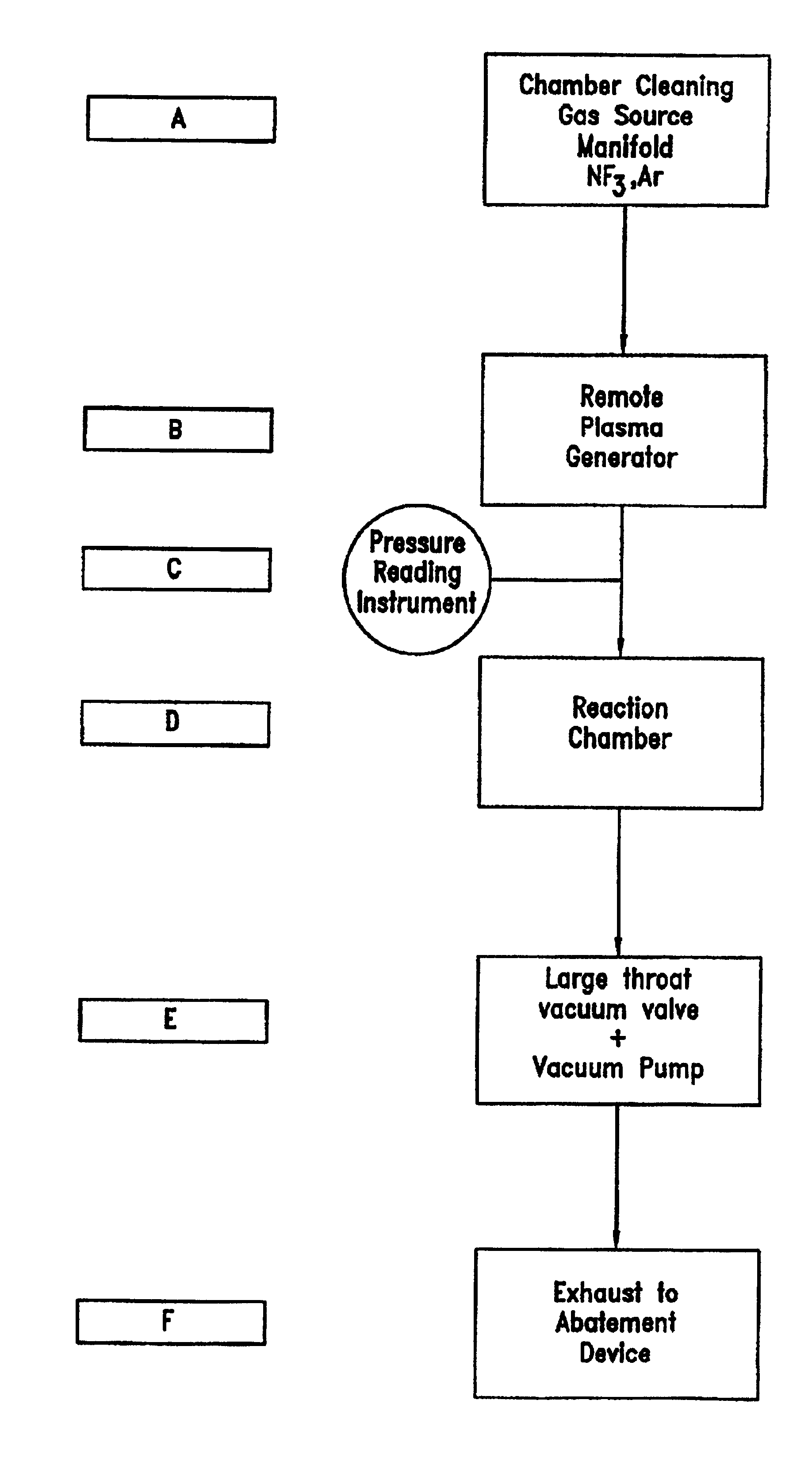
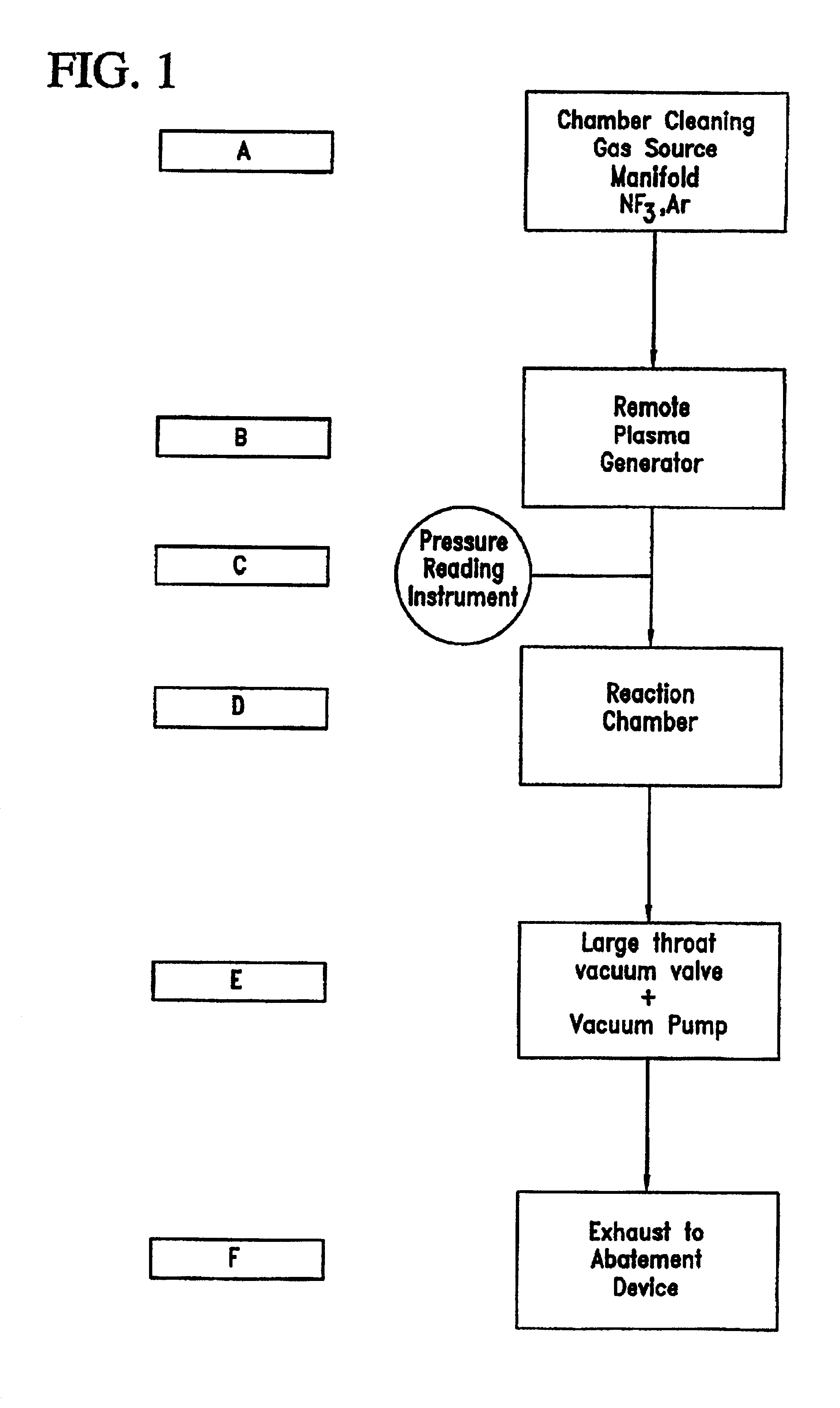
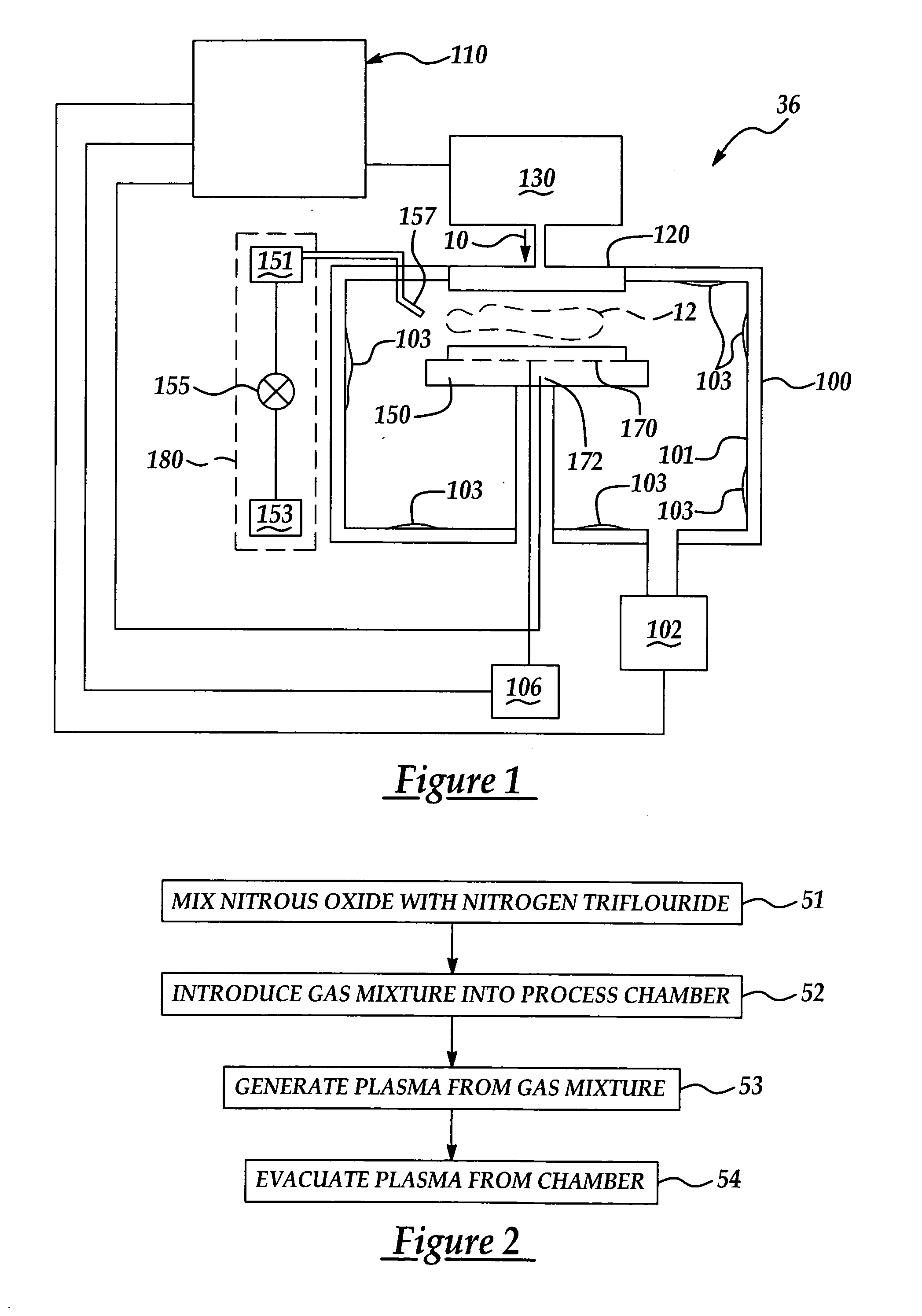
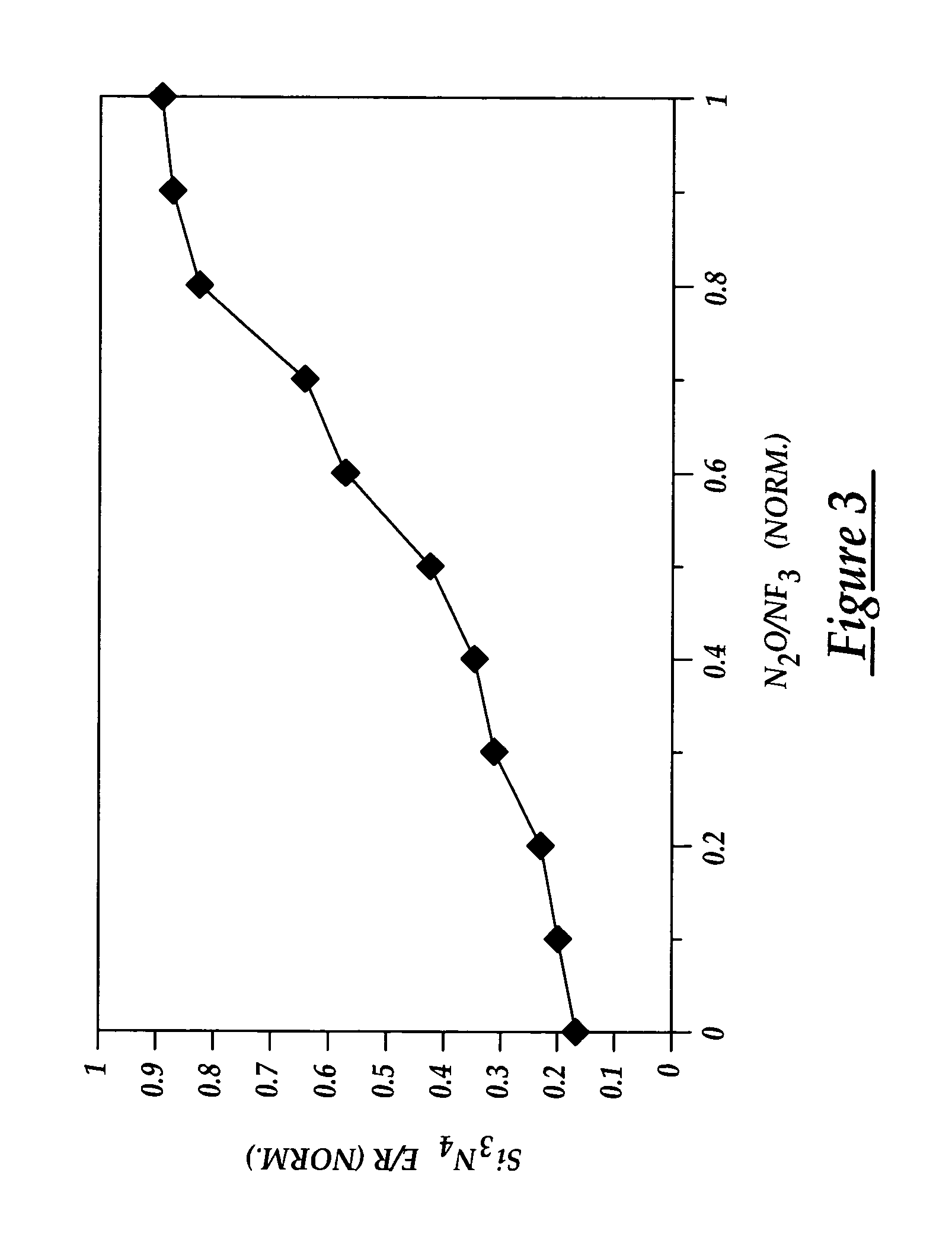
Chamber cleaning method
InactiveUS20050155625A1High densityHigh removal rateHollow article cleaningElectrostatic cleaningNitric oxideVolumetric Mass Density
A method suitable for cleaning the interior surfaces of a process chamber is disclosed. The invention is particularly effective in removing silicon nitride and silicon dioxide residues from the interior surfaces of a chemical vapor deposition (CVD) chamber. The method includes reacting nitrous oxide (N2O) gas with nitrogen trifluoride (NF3) gas in a plasma to generate nitric oxide (NO) and fluoride (F) radicals. Due to the increased density of nitric oxide radicals generated from the nitrous oxide, the etch and removal rate of the residues on the interior surfaces of the chamber is enhanced. Consequently, the quantity of nitrogen trifluoride necessary to efficiently and expeditiously carry out the chamber cleaning process is reduced.
Owner:TAIWAN SEMICON MFG CO LTD
Passivation layer formation by plasma clean process to reduce native oxide growth
Embodiments described herein provide methods for removing native oxide surfaces on substrates while simultaneously passivating the underlying substrate surface. In one embodiment, a method is provided which includes positioning a substrate containing an oxide layer within a processing chamber, adjusting a first temperature of the substrate to about 80° C. or less, generating a cleaning plasma from a gas mixture within the processing chamber, such that the gas mixture contains ammonia and nitrogen trifluoride having an NH3 / NF3 molar ratio of about 10 or greater, and condensing the cleaning plasma onto the substrate. A thin film, containing ammonium hexafluorosilicate, is formed in part, from the native oxide during a plasma clean process. The method further includes heating the substrate to a second temperature of about 100° C. or greater within the processing chamber while removing the thin film from the substrate and forming a passivation surface thereon.
Owner:APPLIED MATERIALS INC
Multicomponent mixed work medium throttling refrigerant applicable to 200-204K refrigerating temperature zone
The multicomponent mixed work medium throttling refrigerating agent applicable to 200-240 k temp. zone includes four groups of substances, respectively are: first group includes tetrafluoromethane, nitrogen trifluoride or mixture; second group includes ethylene, ethane, fluoroform, xenon, fluromethane, perfluoroethylene, fluoroethylene, perfluoroethane or their any two, three and several kinds or mixture of all them; fourth group includes 1-butylene, isobutane, 2-methyl butane, 1-pentene, 3-methyl-1-butylene, 2-methylpentane, 2-butylene, cyclobutane, isobutylene, n-butane, pefluorobutane, n-pentane, perfluoropentane or their two, three, several kinds ormixture of all the them; and third group includees propylene, propane, perfluoropropane, 1,1,1-trifluoroethane, 1,1,-bifluoroethane.
Owner:中科赛凌(北京)科技有限公司
Method for manufacturing a semiconductor device
InactiveUS6864183B2Excellent electrical propertiesSemiconductor/solid-state device detailsSolid-state devicesSulfur hexafluorideMetal silicide
Owner:RENESAS ELECTRONICS CORP
Graphite boat (frame) dry-type cleaning machine
InactiveCN102397859AAvoid mechanical damageEasy to cleanCleaning processes and apparatusPlasma generatorGraphite
The invention relates to a device for maintaining the manufacturing facility of silicon-crystal cell battery, particularly to a graphite boat (frame) dry-type cleaning machine. The graphite boat (frame) dry-type cleaning machine comprises a reaction chamber in which a graphite boat or a graphite frame is disposed. The inlet of the reaction chamber is connected to the NF3 (Nitrogen trifluoride) gas source via an air intake pipe, while the outlet of the reaction chamber is connected to a dry-type vacuum pump via an air exhaust pipe; and said air intake pipe is provided with a plasma generator. The invention is able to remove the silicon nitride on the surface of the graphite boat by processing the reaction of the plasma silicon nitride, therefore the invention is featured in good cleaning effect, simple structure and manufacturing, convenient operation and high cleaning efficiency. Compared with the traditional wet-method chute-type cleaning, the cleaning process can be done without dismantling the graphite boat and external drying; in addition, the cleaning process can be done with higher efficiency and is capable of avoiding mechanical damage for the graphite boat.
Owner:镇江大全太阳能有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com