Purification of nitrogen trifluoride
一种浓度、杂质的技术,应用在获得高纯度三氟化氮领域,能够解决低效率、高经济成本等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
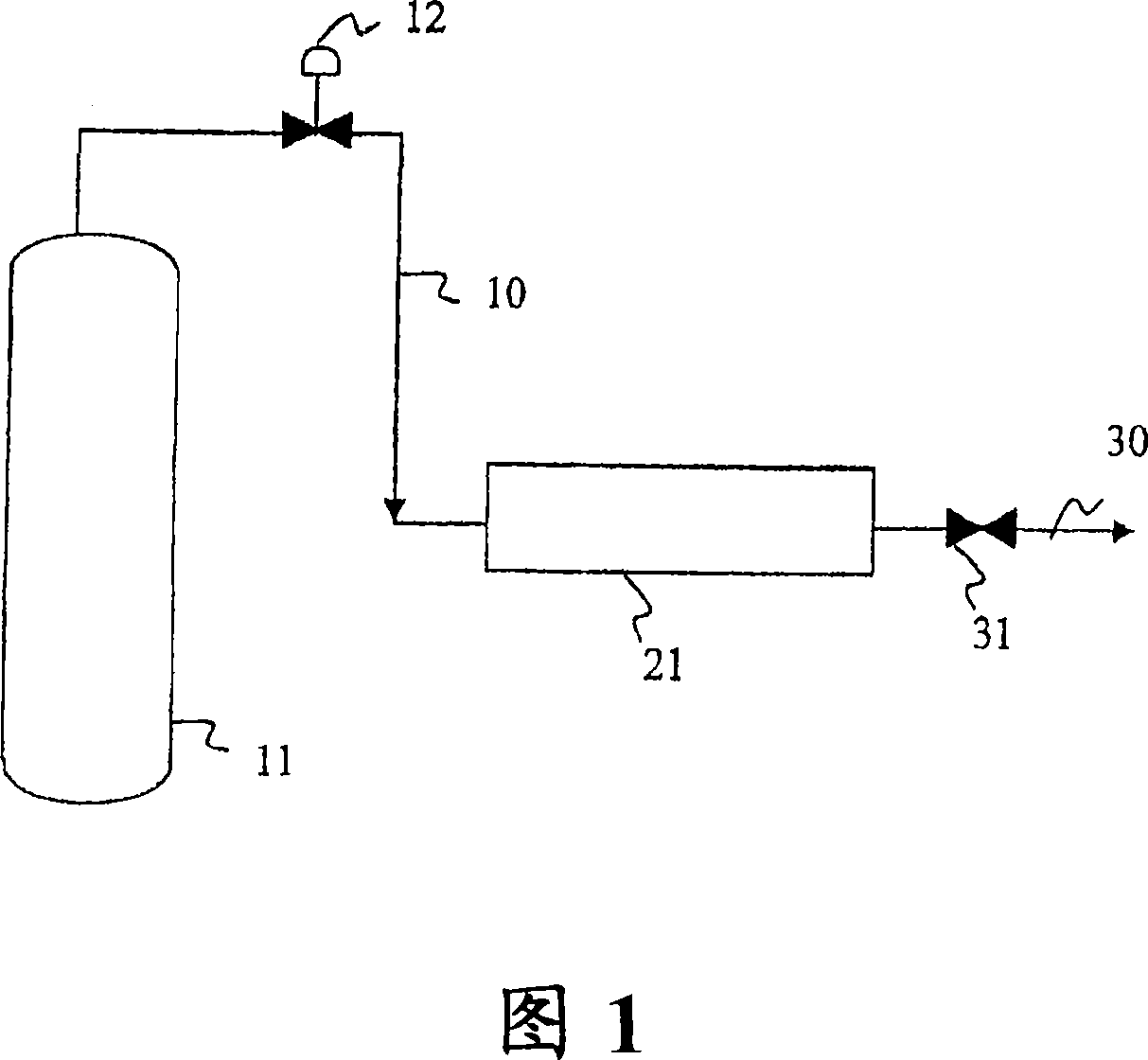
[0019] NF 3 The preparation method generally prepares various levels of CF 4 impurity crude NF 3 flow. crude NF 3 Typically contains from about 100 ppm to about 2% by volume of CF 4 , according to the present invention, this crude product is suitable for purification, although a wide range of concentrations of CF can also be purified by the present invention 4 , eg 4ppm to 5% by volume or higher. It is contemplated that crude products containing any concentration of impurities can be processed in accordance with the claimed invention, provided that appropriate bed sizes and flow rates are utilized.
[0020] The crude product of the present invention can be in any phase including gas, liquid, supercritical phase or some combination of these phases. NF at different temperatures 3 The vapor pressure is well known and will determine the NF being treated 3 phase. For example, at atmospheric pressure (760mm Hg), NF 3 The boiling point is -129.1°C. Therefore, according to ...
Embodiment 1
[0039] Before use, place 10 g of CARBOSPHERE with a particle size of 60 / 80 in a vacuum oven at 100°C Carbon molecular sieve drying. This material was loaded into a 300 mL stainless steel cylinder with NF 3 has about 1995ppmv CF 4 The crude product of impurities was pressurized to about 23 psia. After about 2.3 hours, the gas in the cylinder was analyzed by gas chromatography and found to contain less than 1 ppmv of CF 4 . This example demonstrates that CARBOSPHERE Carbon molecular sieve to CF 4 Compare NF 3 The adsorption is more selective. This example also demonstrates that CARBOSPHERE Molecular sieves can be used to effectively reduce NF 3 CF in gas composition 4 Impurities.
Embodiment 2
[0041] According to the method of Example 1, the difference is that 9.8 grams of CARBOSPHERE Molecular sieves are loaded into the cylinder, and will contain about 2.3% by volume CF at a pressure of about 25 psia 4 NF 3 The crude product was loaded into a cylinder. After about 2.1 hours, the gas in the cylinder was analyzed by gas chromatography and found to contain 0.12% by volume CF 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com