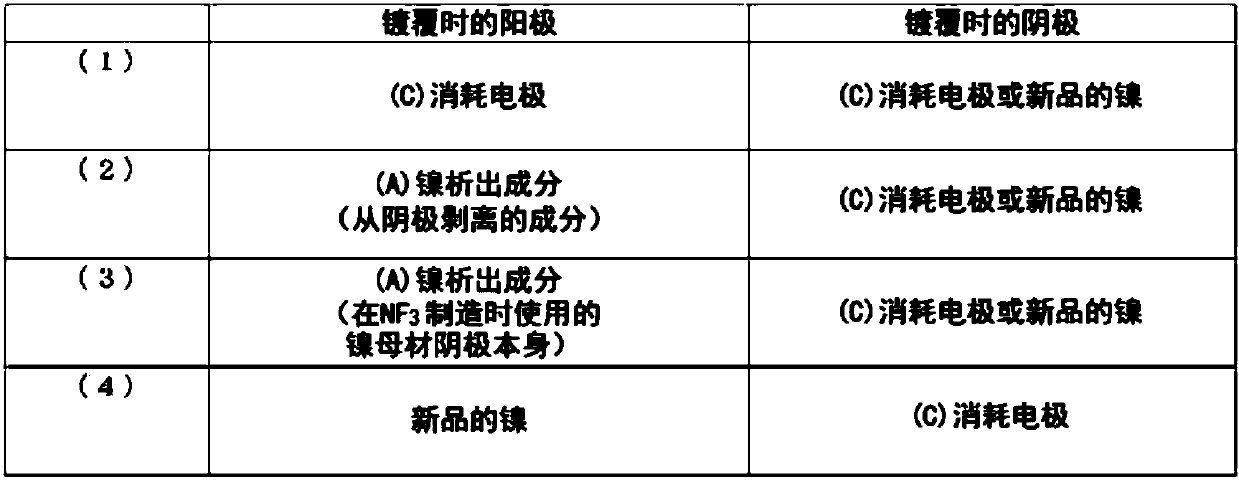
Electrode and production method therefor, and production method for regenerated electrode
A manufacturing method and electrode technology, which can be applied to electrodes, electrolysis components, electrolysis processes, etc., can solve problems such as the decrease of nitrogen trifluoride current efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
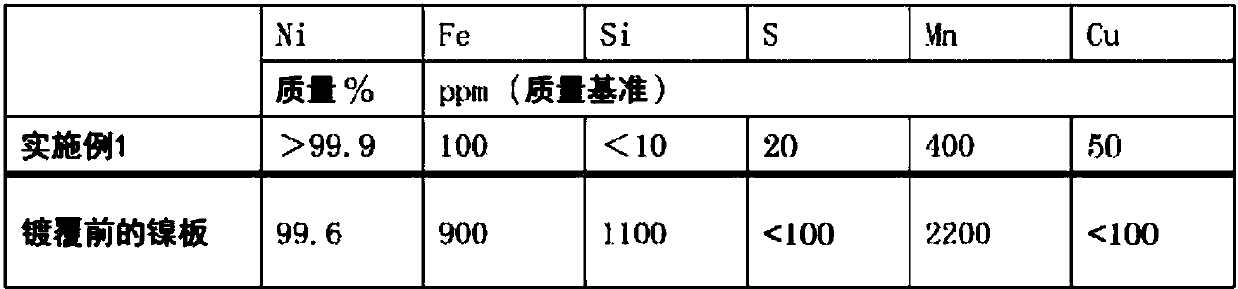
Embodiment 1
[0102] (1) Nickel component recovery (production of nitrogen trifluoride)
[0103] As the anode and the cathode, respectively use a nickel plate with a nickel purity of 99% by mass, and use ammonia and anhydrous hydrofluoric acid to prepare ammonium fluoride-hydrogen fluoride-based molten salt NH in an electrolytic cell. 4 F·1.8HF, the passing temperature is 100℃, the current density is 8A / dm 2 Nitrogen trifluoride was produced with a current efficiency of 65% by molten salt electrolysis under certain conditions. During this production, the nickel component precipitated at the cathode is stripped and recovered. The area of the anode after nitrogen trifluoride production is 70% or more of the electrode area before use.
[0104] (2) Manufacture of regenerative electrodes
[0105] 170 g of nickel sulfate hexahydrate, 32 g of nickel chloride hexahydrate, and 21 g of boric acid were dissolved in 0.7 L of pure water to prepare a plating bath. As the cathode, a nickel electrode...
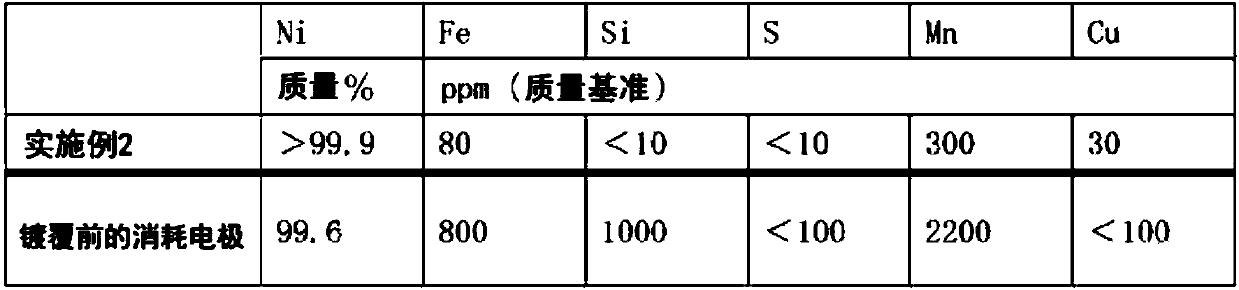
Embodiment 2
[0112] (1) Nickel component recovery (production of nitrogen trifluoride)
[0113] As the anode and cathode, nickel electrodes with a nickel purity of 99% by mass were used, and ammonium fluoride-hydrogen fluoride-based molten salt NH was prepared in an electrolytic cell using ammonia and anhydrous hydrofluoric acid. 4 F·1.8HF, the passing temperature is 120°C, the current density is 5A / dm 2 Nitrogen trifluoride was produced with a current efficiency of 65% by molten salt electrolysis under certain conditions. During this production, the nickel component precipitated at the cathode is stripped and recovered. The area of the anode after nitrogen trifluoride production is 50% or more of the electrode area before use.
[0114] (2) Manufacture of regenerative electrodes
[0115] 36.1 kg of nickel sulfate hexahydrate, 7.1 kg of nickel chloride hexahydrate, and 5.0 kg of boric acid were dissolved in pure water in a 150 L electrolytic cell whose inner surface was coated with Tef...
Embodiment 3
[0122] (1) Nickel component recovery (production of nitrogen trifluoride)
[0123] It operates similarly to Example 2.
[0124] (2) Manufacture of regenerative electrodes
[0125] Instead of the recovered nickel component used in (2) of Example 2, the cathode itself used in the manufacture of nitrogen trifluoride in (1) of Example 3 was used for the anode as nickel for dissolution. A regenerative electrode was fabricated under the same conditions as in Example 2. The resulting regenerative electrode has a mass increased by 90% relative to the consumable electrode used as a cathode. As a result of measurement by ion chromatography under the above conditions, the fluoride ion concentration in the plating bath at the end time was 0.8 g / L. Analysis of a part of the produced regenerative electrode by X-ray diffraction revealed that it was metallic nickel, and no fluorine components such as nickel fluoride were detected. As a result of analysis using the X-ray microanalyzer (acc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com