Method for nitrogen trifluoride production
A technology of nitrogen trifluoride and perfluorocarbon, applied in the direction of nitrogen trifluoride, hydrogen fluoride, fluorine/hydrogen fluoride, etc., can solve the problems of toxicity, high production cost of fluorine, corrosion of metal reactors and components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
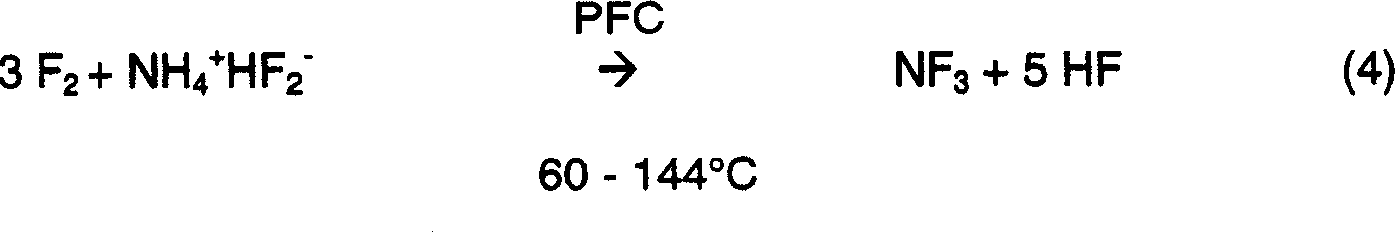
[0040] Example 1: Fomblin at 96°C _ 25 / 6 Ammonium bifluoride in PFC solvent
[0041] In a 300-cc Monel manufactured by Parr Instrument Company of Moline, IL _ Add 75 grams (1.32m) of solid ammonium bifluoride (NH 4 + HF 2 - ) (referred to herein as ABF) and approximately 150 mL (286 g) of Fomblin _ 25 / 6 oil formed the reaction mixture. The reactor is equipped with cooling coils or baffles, thermocouple probes, F 2 Feed tube, into liquid NH 3 Feed tube, pressure gauge, exhaust port and gas propeller type agitator. The mixture was stirred at a speed greater than 1900 revolutions per minute (rpm) while externally heating the mixture to a working temperature of about 96°C. Once the operating temperature is reached, 100% concentration of fluorine gas is introduced into the mixture at a rate of 50 standard cubic centimeters (sccm) per minute (sccm), or about 2.2 mmol / min, through the inlet pipe, and is discharged directly from the reactor through the exhaust port product. ...
Embodiment 2
[0044] Example 2: In Fomblin _ 25 / 6 PFC solvent at 96°C - high F 2 ammonium bifluoride
[0045] Nitrogen trifluoride was prepared as described in Example 1, except that 75.4 grams (1.32 mol) of solid ABF and about 150 mL (289 g) of Fomblin _ The 25 / 6 oil was mixed in a Monel stirred reactor. Once at operating temperature, set F 2 (100% concentration) was fed into the stirred mixture through the dip tube at a rate of 73.3 sccm and the gaseous product exited the reactor directly through the opening in the headspace. On exiting the reactor, the gaseous products were treated and analyzed as described in Example 1. The fluorine feed was continued for approximately 260 minutes, and the composition of the gaseous products exiting the reactor was measured approximately every 20 minutes. After the first 180 minutes of reaction time, vent the reactor of fluorine, NF 3 , N 2 and N 2 The concentration of O reaches a "steady state" concentration. Under "steady state" conditions, t...
Embodiment 3
[0056] Example 3: In Fomblin _ Ammonium bifluoride in 25 / 6 PFC solvent at 115°C
[0057] This experiment was carried out as described in Example 1, except that 100.2 grams (1.76 mol) of solid ABF and about 200 mL (364 g) of Fomblin _ The 25 / 6 oil was mixed in a Monel stirred reactor. The mixture was heated externally until a working temperature of 114-115°C was reached. Once at operating temperature, set F 2 (100% concentration) was fed into the stirred mixture through a dip tube at a rate of 50 sccm and the gaseous product exited the reactor directly through an opening in the headspace. On exiting the reactor, the gaseous products were treated and analyzed as described in Example 1. At "steady state" the fluorine conversion was 98%. The normalized gaseous product composition is 94% NF 3 , 5%N 2 , and 1% N 2 O. NF 3 The yield is 92%.
[0058] Compared with Example 1, when the working temperature increases, NF 3 Yield decreased slightly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com