Macromolecular polymerizable photoinitiator and preparation thereof
A technology for polymerizing photoinitiators and photoinitiators is applied in the field of preparation of macromolecular photoinitiators. Simple steps and operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
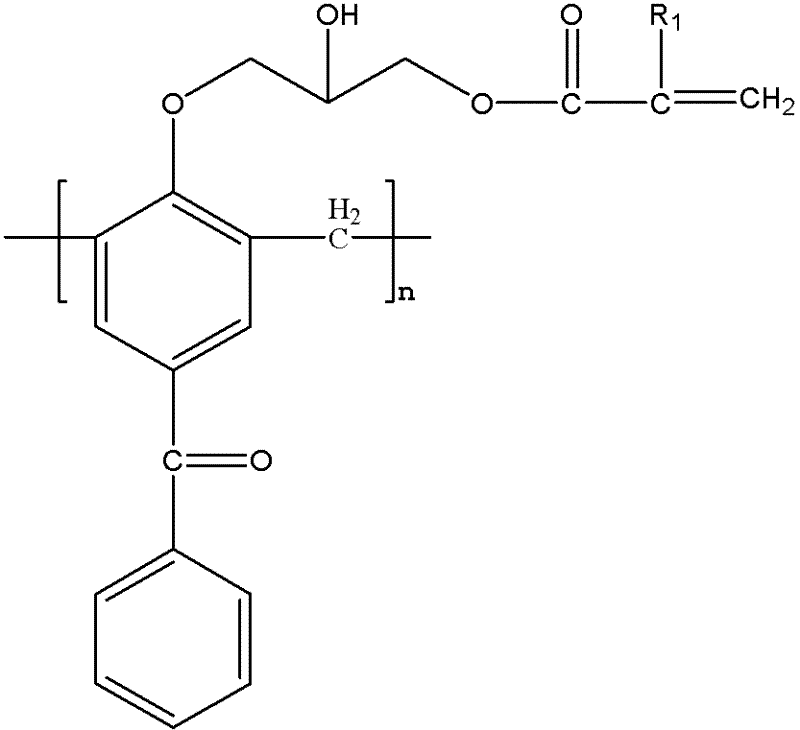
[0023] Synthesis of Hydrogen-Substituted Macromolecular Polymerizable Photoinitiators
[0024] Add 10g of 4-hydroxybenzophenone and 4.5g of formaldehyde solution into a three-necked flask, add 0.44g of sodium hydroxide, heat and stir to 95°C, and react for 2h; After the end, cool to 105°C to collect the product, pour the product into water to precipitate, and obtain a light yellow macromolecular photoinitiator containing benzophenone after suction filtration. The obtained light yellow macromolecular photoinitiator containing benzophenone is dissolved in 21.2g methylene chloride, 0.4g boron trifluoride is added, 5.6g epichlorohydrin is dissolved in 11g methylene chloride, within 3 hours Slowly add the mixed solution dropwise to the three-necked flask, the reaction temperature is 0-5°C, after the dropwise addition, stir at 70°C for 3 hours, use a rotary evaporator to remove unreacted epichlorohydrin and dichloromethane from the mixed solution; use 40g ethanol Dissolve the resid...
Embodiment 2
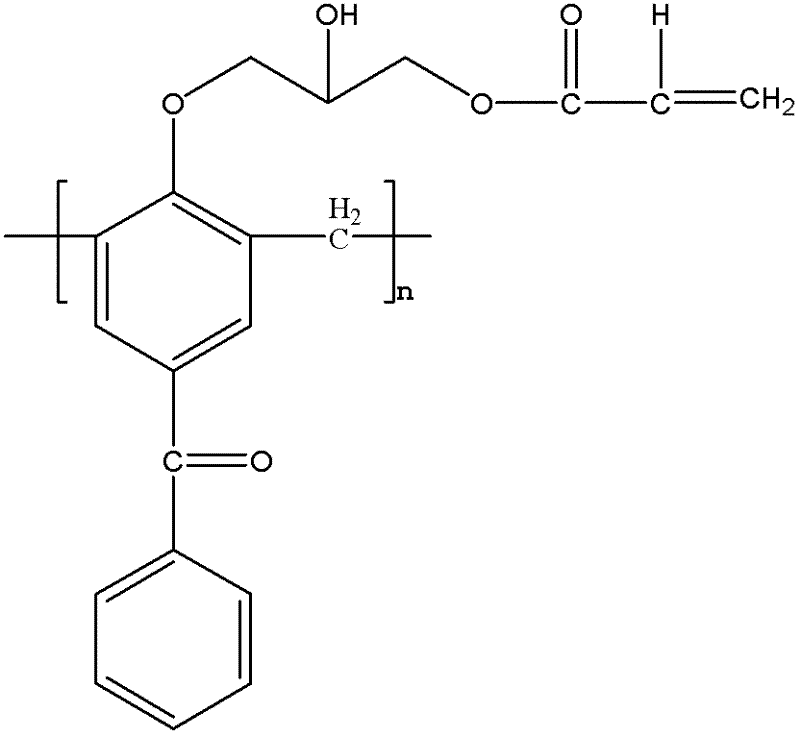
[0028] Synthesis of Methyl Substituted Macromolecular Polymerizable Photoinitiators
[0029] Add 10g of 4-hydroxybenzophenone and 4.5g of formaldehyde solution into a three-necked flask, add 0.44g of sodium hydroxide, heat and stir to 95°C, and react for 2h; After the end, cool to 105°C to collect the product, pour the product into water to precipitate, and obtain a light yellow macromolecular photoinitiator containing benzophenone after suction filtration. The obtained light yellow macromolecular photoinitiator containing benzophenone is dissolved in 22g of methylene chloride, 0.4g of boron trifluoride is added, 5.6g of epichlorohydrin is dissolved in 13g of methylene chloride, slowly within 3 hours Add the mixed solution dropwise to the three-necked flask, the reaction temperature is 0-5°C, after the dropwise addition, stir at 70°C for 3 hours, use a rotary evaporator to remove unreacted epichlorohydrin and dichloromethane from the mixed solution; use 40g of ethanol to disso...
Embodiment 3
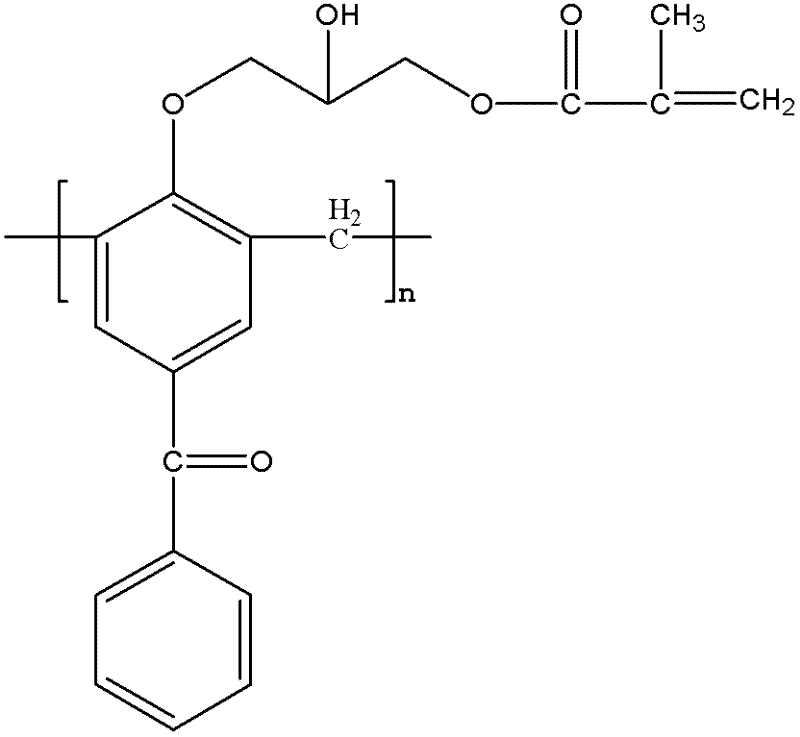
[0033] Synthesis of Methoxyl Substituted Macromolecular Polymerizable Photoinitiators
[0034]Add 10g of 4-hydroxybenzophenone and 4.6g of formaldehyde solution into a three-necked flask, add 0.5g of sodium hydroxide, heat and stir to 95°C, and react for 2h; After the end, cool to 105°C to collect the product, pour the product into water to precipitate, and obtain a light yellow macromolecular photoinitiator containing benzophenone after suction filtration. The obtained pale yellow macromolecular photoinitiator containing benzophenone is dissolved in 21g of methylene chloride, 0.4g of boron trifluoride is added, 5.6g of epichlorohydrin is dissolved in 15g of methylene chloride, slowly within 3 hours Add the mixed solution dropwise to the three-necked flask, the reaction temperature is 0-5°C, after the dropwise addition, stir at 70°C for 3 hours, use a rotary evaporator to remove unreacted epichlorohydrin and dichloromethane from the mixed solution; use 40g of ethanol to dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com