Method of preparing waterless lithium terafluoroborate
A technology of lithium tetrafluoroborate and tetrafluoroborate, which is applied to the preparation of lithium ion battery electrolyte anhydrous lithium tetrafluoroborate and the preparation of lithium salt, can solve the problem of reducing the purity of lithium tetrafluoroborate, affecting the effect of dehydration, and increasing cost and other problems, to achieve the effect of complete crystal form, improved purity and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
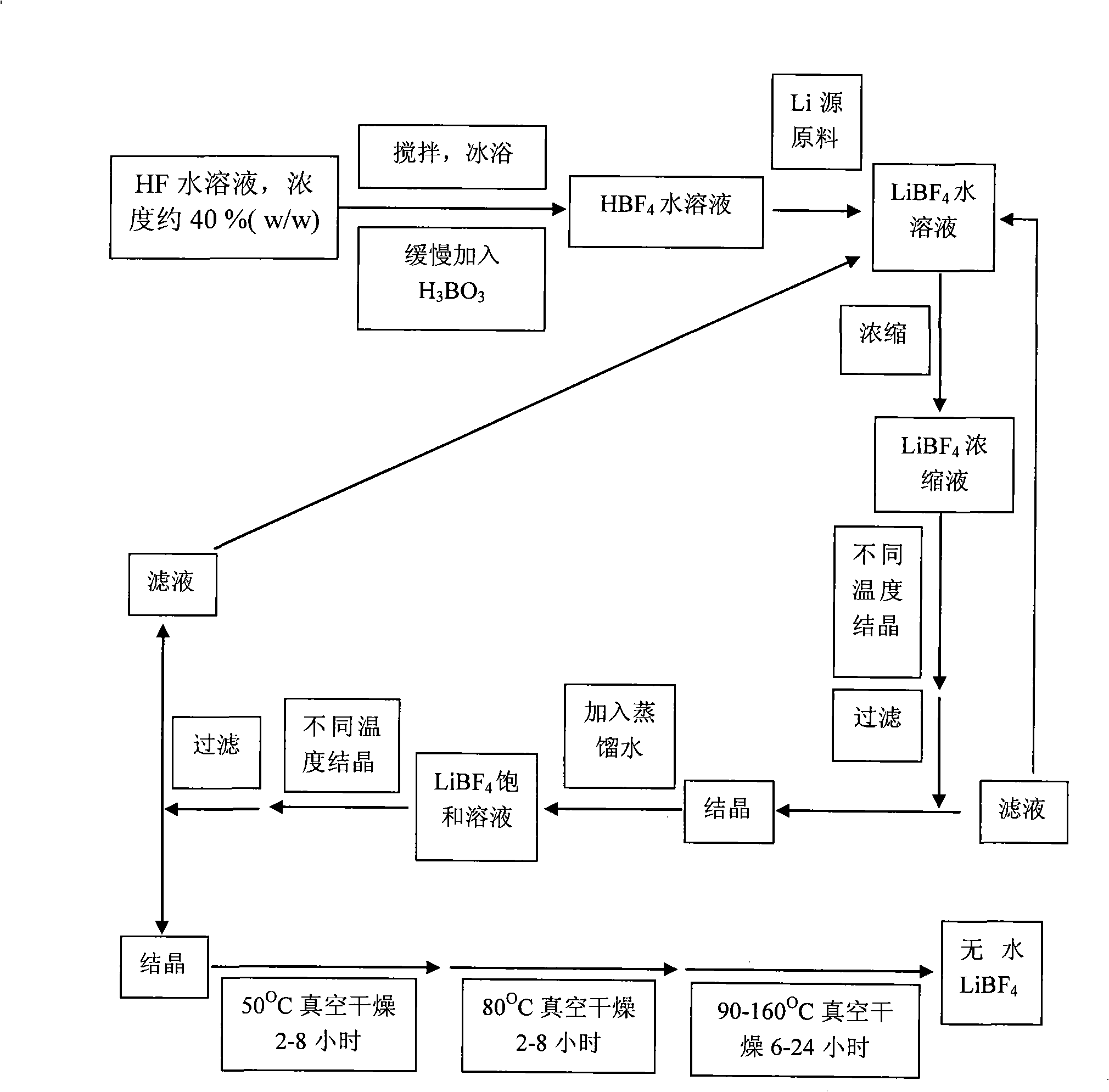
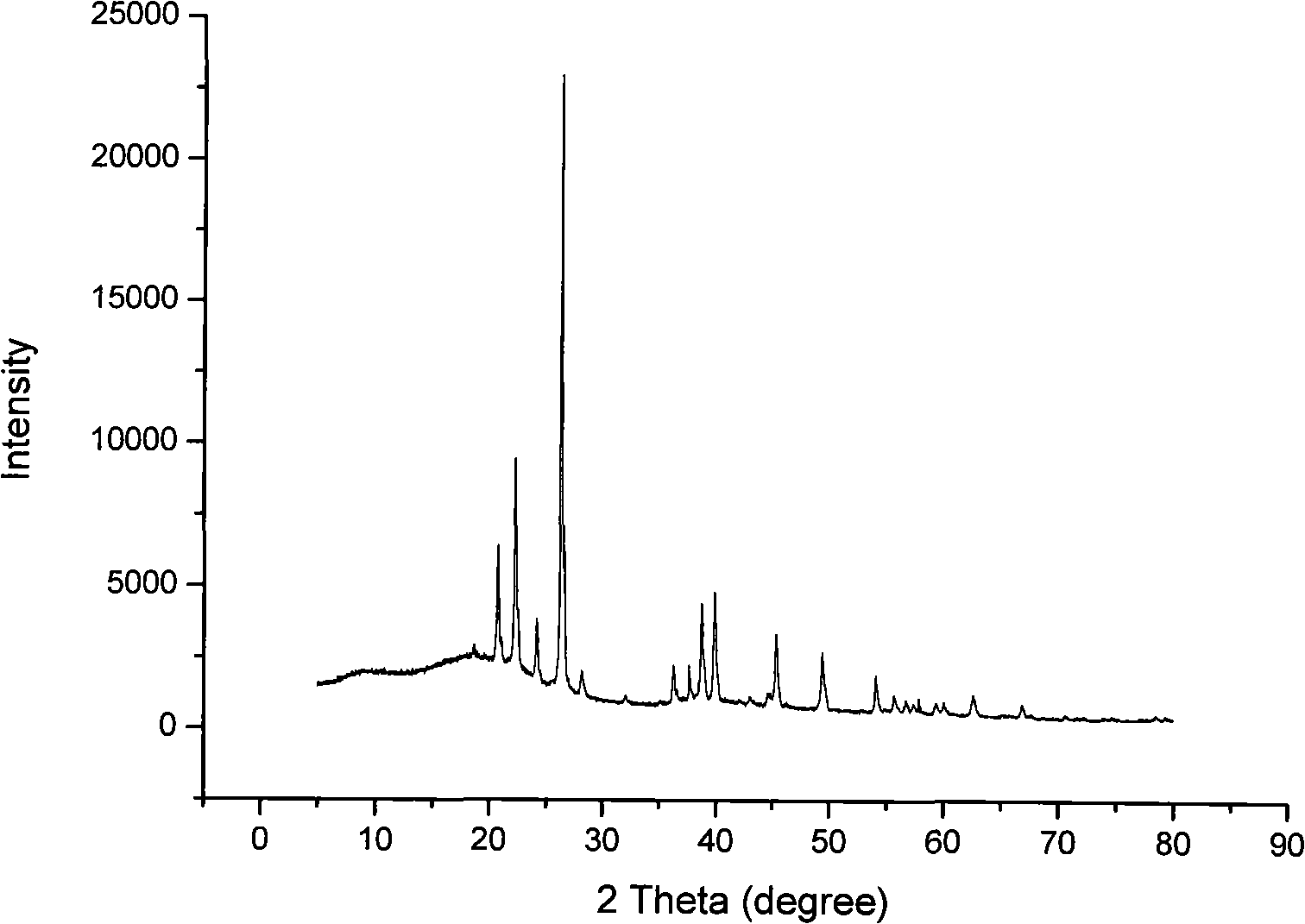
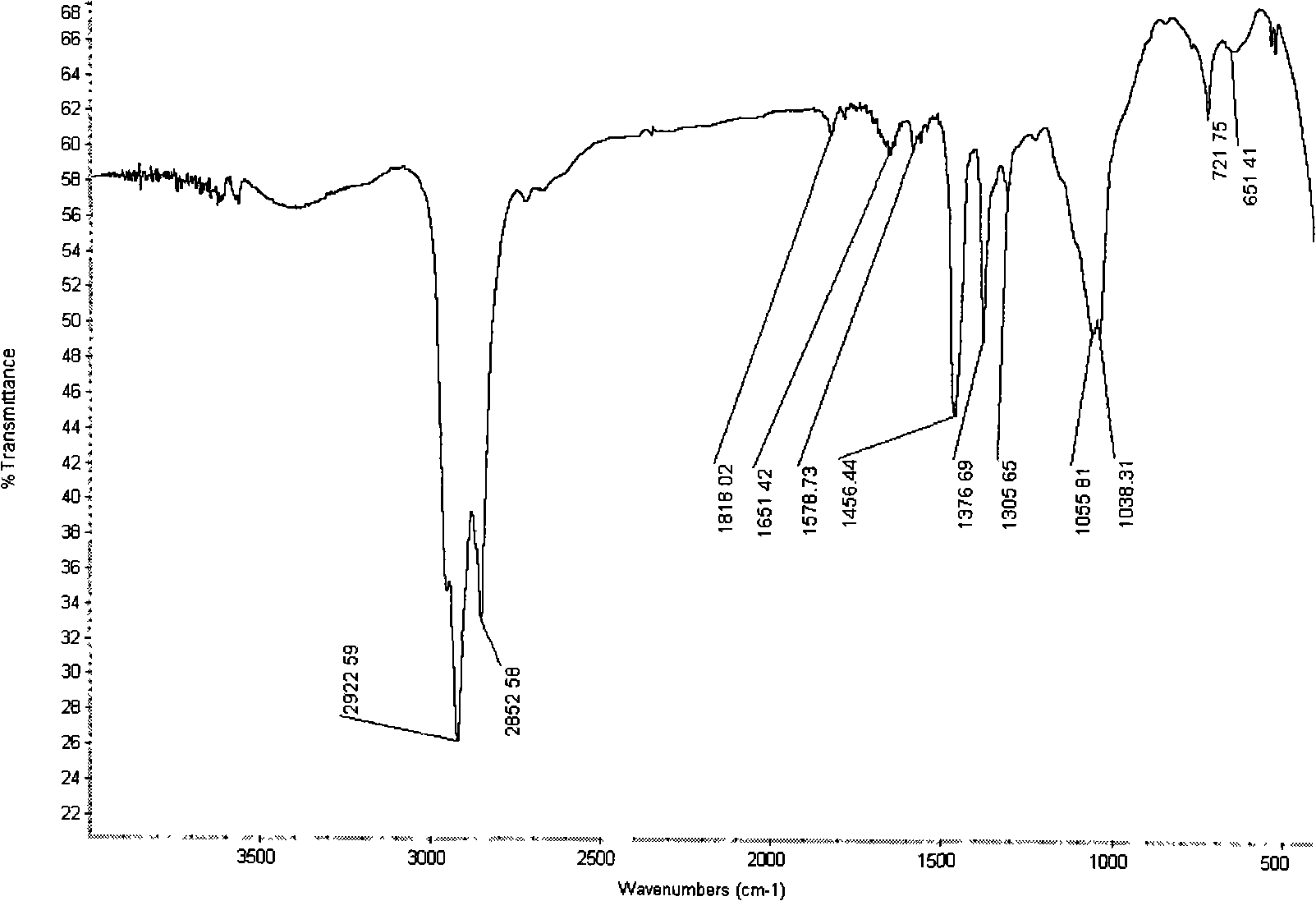
[0021] Weigh the mass fraction to be about 40% (using the national standard GB620-93 method to calibrate the purchased analytical pure hydrofluoric acid, its mass fraction is about 40.87%, and the mass fraction of hydrofluoric acid is generally said to be 48 when using hydrofluoric acid in foreign literature. %, the same below) 209g of hydrogen fluoride aqueous solution was placed in an ice bath and stirred with a magnetic stirrer. After 10 minutes, 66.1g of boric acid was slowly added and the addition was completed in about 50 minutes. After the addition, continue to stir for 30 minutes, then slowly add 40.6g of lithium carbonate to the above mixed solution, add in about 30 minutes, filter, and concentrate the filtrate under an infrared lamp to make the content of lithium tetrafluoroborate about 70% . Put the concentrated solution in a constant temperature water bath at about 40°C, filter it after 12 hours, and return the filtrate to the mother liquor. The solid phase is dissolve...
Embodiment 2
[0023] Weigh 104.4 g of hydrogen fluoride aqueous solution with a mass fraction of about 40%, put it in an ice bath, and stir with a magnetic stirrer. After 10 minutes, slowly add 33 g of boric acid and complete the addition in about 50 minutes. After the addition, continue to stir for 30 minutes, then slowly add 20.3g of lithium carbonate to the above mixed solution, add in about 30 minutes, filter, and concentrate the filtrate under an infrared lamp to make the content of lithium tetrafluoroborate about 70% . Put the concentrated solution in a constant temperature water bath at about 5°C, filter after 2 hours, and return the filtrate to the mother liquor. The solid phase is placed in a vacuum drying oven. Dry for 5 hours at about 50°C and 560mmHg, then heat to about 80°C, continue drying for 5 hours, remove the solid from the vacuum drying oven, grind, and then put it in the vacuum drying oven again, and dry at about 140°C and 560mmHg for 10 Within hours, white powdery anhydrous...
Embodiment 3
[0025] Weigh 105 g of hydrogen fluoride aqueous solution with a mass fraction of about 40%, put it in an ice bath, and stir with a magnetic stirrer. After 10 minutes, slowly add 33 g of boric acid and complete the addition in about 50 minutes. After adding, continue to stir for 30 minutes, then add 20.9gLiOH·H 2 O was slowly added to the above mixed solution, the addition was completed in about 30 minutes, filtered, and the filtrate was concentrated under an infrared lamp so that the content of lithium tetrafluoroborate was about 70%. Put the concentrated solution in a constant temperature water bath at about 40°C, filter it after 12 hours, and return the filtrate to the mother liquor. The solid phase is dissolved with an appropriate amount of distilled water to dissolve the crystals exactly, and the solution is again placed in a constant temperature water bath at about 30°C. After 72 hours, it is filtered, the filtrate is returned to the mother liquor, and the solid phase is plac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com