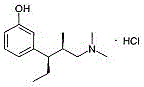
Preparation method of tapentadol intermediate
A technology for tapentadol and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, and can solve problems such as difficult large-scale application, low utilization rate of raw materials, and increased costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0049] (1) Take magnesium chips (0.1 mol) in a 100 mL round bottom flask, add a small amount of iodine, under nitrogen protection, add 10 mL of anhydrous tetrahydrofuran and 0.05 mol of the compound of formula (VI), react for 15 minutes, continue to dropwise add 0.095 mol of 80ml of anhydrous THF of the compound of formula (VI), until the end of the reaction, the Grignard reagent of the compound of formula (VI) is obtained. The Grignard reagent can also be prepared according to other methods in the prior art, which does not affect the spirit of the present invention.
[0050] (2) In a 100ml flask, add 0.01mmol of S-BINOL, under the protection of nitrogen, add 25ml of anhydrous tetrahydrofuran, dropwise add 0.05mmol of titanium tetraisopropoxide, and react for 60min. Add dropwise 1.5 mmol of the Grignard reagent synthesized in step (1) (dissolved in 30 ml of anhydrous tetrahydrofuran) under an ice bath, continue the reaction for 1.5 hours, and dropwise add 1.3 mmol of the compo...
preparation Embodiment 2
[0064] The pilot scale test was carried out according to the method described in Preparation Example 1, except that the addition of each raw material was increased to 1000 times that of the raw materials described in Preparation Example 1. Wherein the yield of step (2) is 80%, the yield of step (3) is 89%, the yield of step (4) is 90%, and the yield of step (5) is 82%. The single impurity of the compound of the obtained formula (I) is ≤0.14 wt%, and the total impurity is ≤0.2 wt%; after refining with ethanol-water, the content is ≥99%, which meets the requirements of the quality standard of this product in European Pharmacopoeia EP-7.0, and the total impurity<0.1 %.
[0065] The chromatographic detection conditions and method are the same as those in Preparation Example 1.
[0066] The invention has the following technical effects: the reactions in each step are preferably carried out at a relatively low temperature, the reaction conditions are mild, the synthesis of impuriti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com