Method for synthesizing substituted indole compounds through one-pot method
A technology for indoles and compounds, which is applied in the field of one-pot synthesis of substituted indole compounds, can solve the problems of expensive catalysts, high reaction temperature, and difficulty in industrialization, and achieve the effects of simple operation, mild reaction conditions, and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
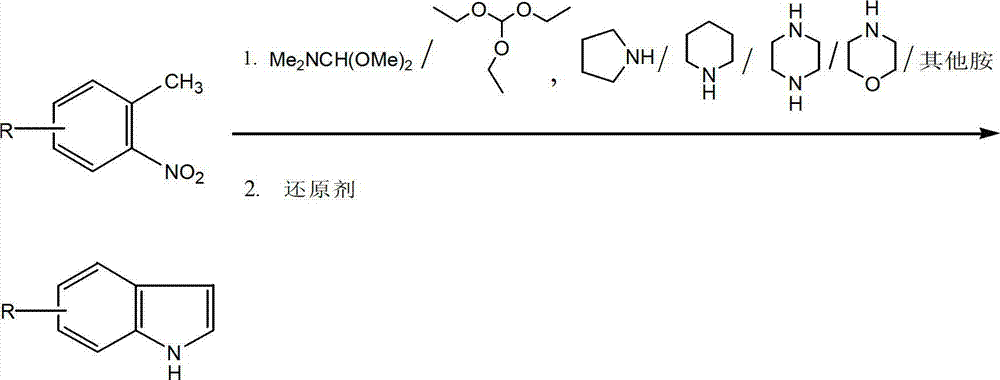
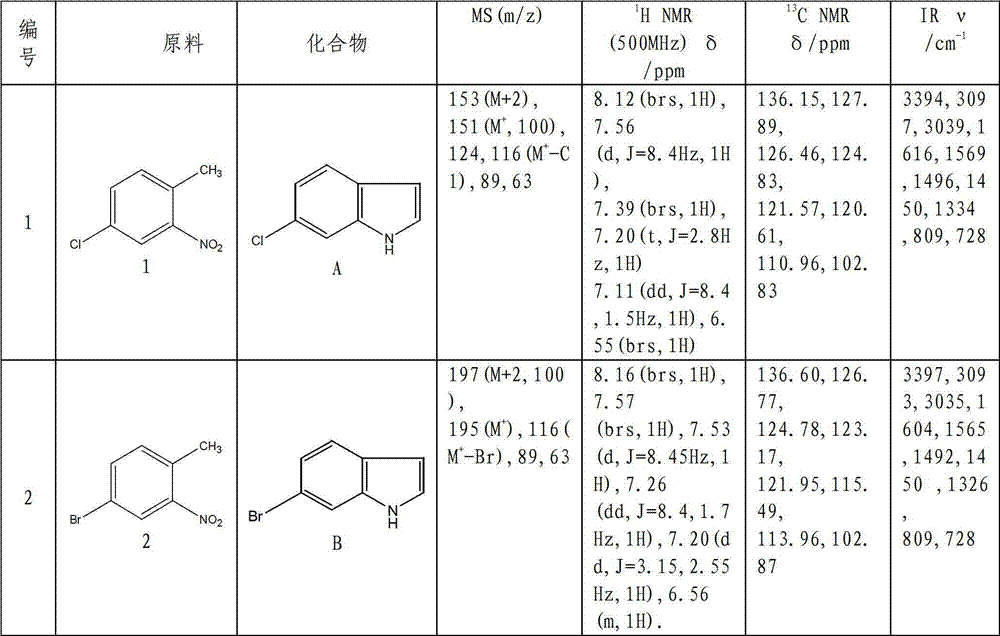
[0028] Compound A: In a 100ml three-necked flask, add 50ml m-trimethylbenzene, raw material 1 (4-chloro-2-nitrotoluene, the same below) (0.69g, 4mmol), N,N-dimethylformamide dimethyl acetal (0.95g, 8mmol), pyrrolidine (1.14g, 16mmol), under nitrogen protection, stir well to completely dissolve the substrate. The temperature was raised to 145° C., condensed to reflux, and the reaction process was tracked by TLC until thin-layer chromatography showed that the starting point disappeared, and then cooled to room temperature. Then add a catalytic amount (20mg) of palladium carbon, and pass through hydrogen (10ml / min), control the reaction temperature at 45-50°C, react for 2 hours, cool to room temperature, filter with suction, and rinse the filter residue with dichloromethane several times. Extraction and recrystallization (cyclohexane:dichloromethane:methanol=2:1:8 (v / v)) gave an off-white solid with a yield of 92.3%.
[0029] The preparation method of compound B-H is the same as...
Embodiment 2
[0031] Compound A: In a 100ml three-necked flask, add 50ml N,N-dimethylformamide (DMF), raw material 1 (0.69g, 4mmol), N,N-dimethylformamide dimethyl acetal (0.95g, 8mmol ), pyrrolidine (1.14g, 16mmol), under nitrogen protection, and fully stirred to completely dissolve the substrate. The temperature was raised to 130° C., condensed to reflux, and the reaction process was tracked by TLC until thin-layer chromatography showed that the raw material point disappeared, and then cooled to room temperature. Then add a catalytic amount (100mg) of Raney nickel, slowly drop in 85% hydrazine hydrate (0.35g, 6mmol) with a dropping funnel, raise the temperature to 30°C, add the same amount of hydrazine hydrate every 30 minutes, and control the reaction temperature At 45-50°C, follow TLC until thin layer chromatography shows that the component point of the intermediate product disappears, cool to room temperature, and remove the catalyst by filtration with celite (do not drain to prevent t...
Embodiment 3
[0034] Compound A: Add 50ml tetrahydrofuran (THF), raw material 1 (0.69g, 4mmol), N,N-dimethylformamide dimethyl acetal (0.95g, 8mmol), pyrrolidine (1.14g, 16mmol), under nitrogen protection, fully stirred to dissolve the substrate completely. Raise the temperature to 80°C, condense to reflux, track the reaction process with TLC, until thin layer chromatography shows that the raw material point disappears, and cool to room temperature. Then add ferrous acetate (1.4g, 8mmol), control the reaction temperature at 45-50°C, track with TLC, until thin layer chromatography shows that the component point of the intermediate product disappears, cool to room temperature, filter with suction, and filter the residue with dichloromethane Wash several times, extract and recrystallize (cyclohexane:dichloromethane:methanol=2:1:8) to obtain an off-white solid with a yield of 78.5%.
[0035] Using raw materials 2-14 instead of raw material 1, the feed ratio is the same as that of compound 1, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com