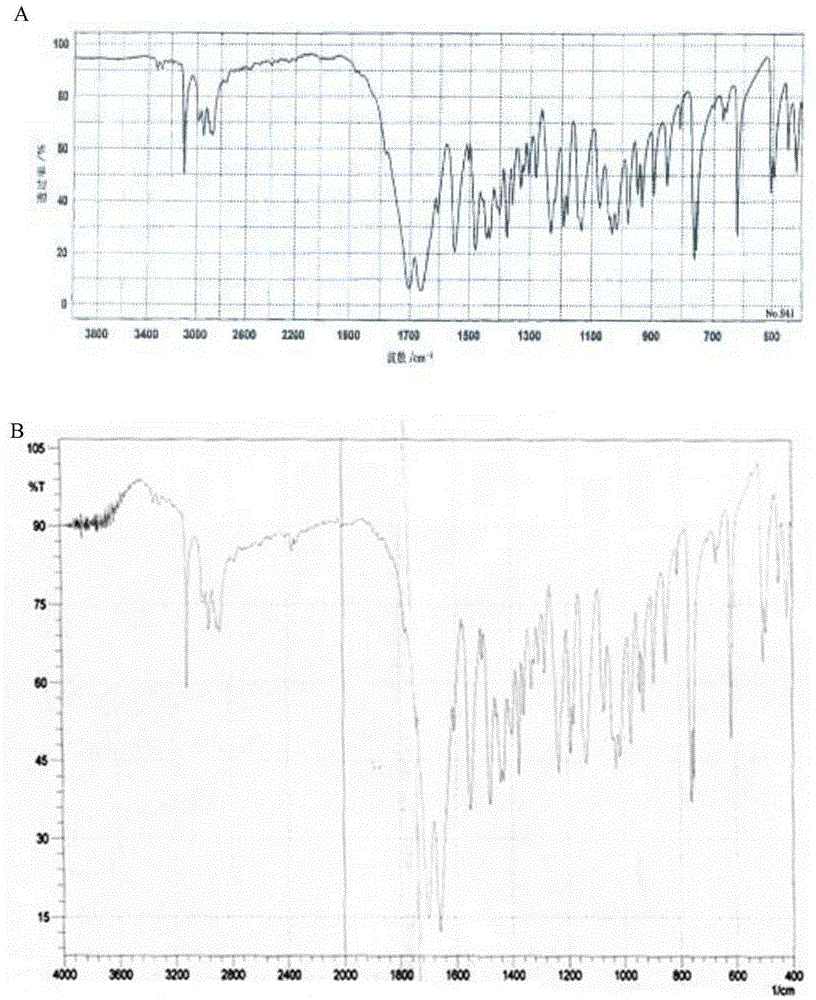
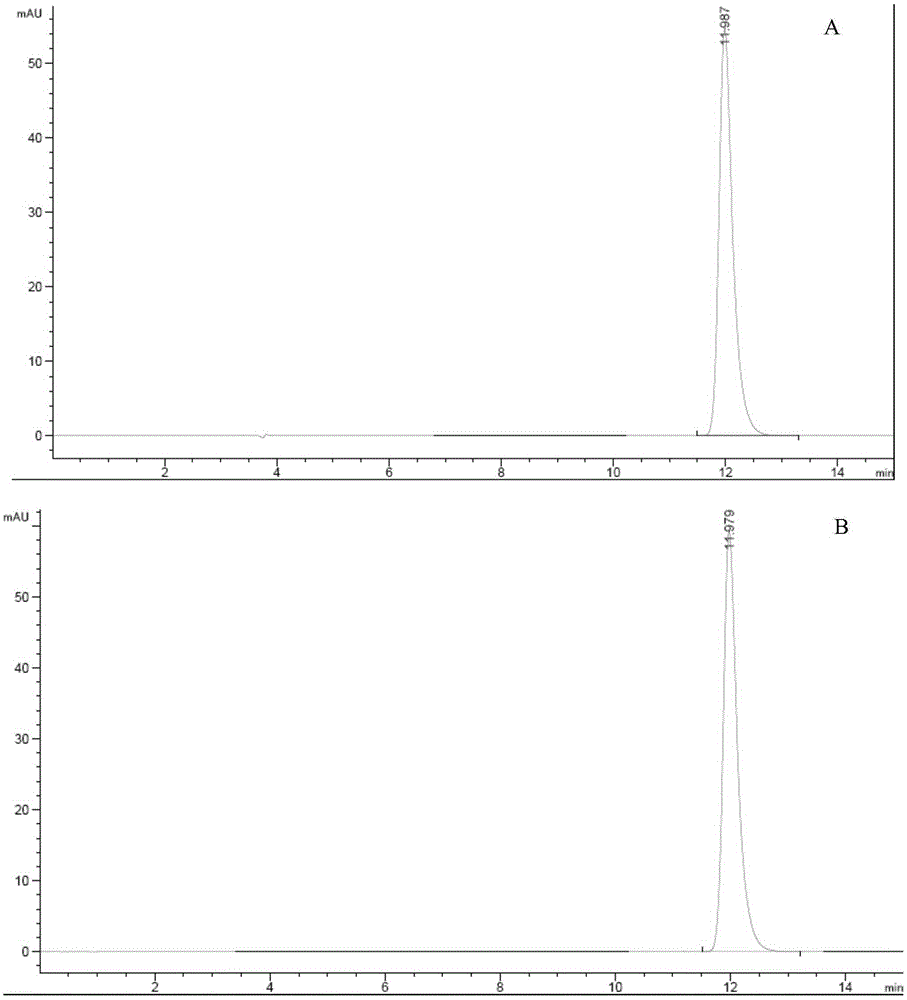
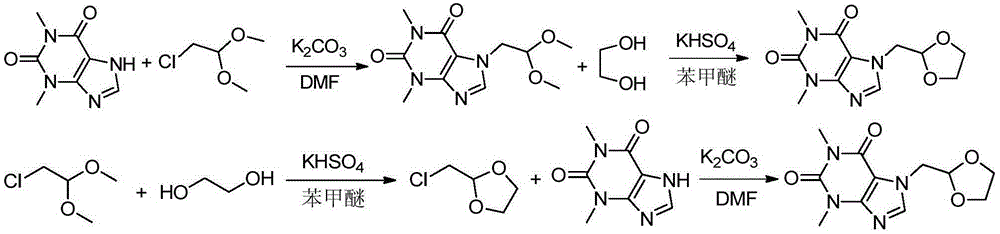
Preparation methods of doxofylline
A technology of doxofylline and theophylline, which is applied in the field of preparation of doxofylline, can solve problems such as easy residue, benzene residue, high genotoxicity, etc., achieve mild and easy-to-control reaction conditions, solve toxic residues, and yield of finished products high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of doxofylline, comprising:
[0036] (1) Take 20g of theophylline, 15.4g of anhydrous potassium carbonate, 14.5g of chloroacetaldehyde dimethyl acetal, and 2.8g of tetrabutylammonium bromide, add them to 120mL of dimethylformamide, and heat up to 145°C for reaction until theophylline basically reacts, stop the reaction, lower the temperature, filter, evaporate the filtrate to dryness under reduced pressure, and recrystallize the ethanol with a mass fraction of 95%, to obtain the intermediate 7-(2,2-dimethoxyethyl)tea alkali. The mass of the intermediate 7-(2,2-dimethoxyethyl)theophylline is 21g.
[0037] (2) Add 20g of 7-(2,2-dimethoxyethyl)theophylline, 9.3g of ethylene glycol, and 2.3g of potassium bisulfate to 150ml of anisole, and heat the oil bath to 115°C until the basic reaction of the raw materials Complete, stop heating, lower the temperature, pour out the supernatant, and evaporate the supernatant to dryness under reduced pressure to obt...
Embodiment 2
[0040] A preparation method of doxofylline, comprising:
[0041] (1) Take 200g of theophylline, 129.4g of anhydrous sodium carbonate, 152.1g of chloroacetaldehyde dimethyl acetal, and 15.9g of tetrabutylammonium bromide, add them to 800mL of dimethylformamide, and heat up to 140°C for reaction until theophylline basically reacts, stop the reaction, lower the temperature, filter, evaporate the filtrate to dryness under reduced pressure, and recrystallize the ethanol with a mass fraction of 95%, to obtain the intermediate 7-(2,2-dimethoxyethyl)tea alkali. The mass of the intermediate 7-(2,2-dimethoxyethyl)theophylline is 225g.
[0042] (2) Take 210g intermediate 7-(2,2-dimethoxyethyl)theophylline, 74.5g ethylene glycol, 33.5g potassium bisulfate and add it to 1100ml anisole, and the oil bath is heated to 130°C until After the raw materials have basically reacted, stop heating, lower the temperature, pour out the supernatant, and evaporate the supernatant to dryness under reduc...
Embodiment 3
[0044] A preparation method of doxofylline, comprising:
[0045](1) Take 200g of theophylline, 199.1g of anhydrous potassium carbonate, 179.9g of chloroacetaldehyde dimethyl acetal, and 17.9g of tetrabutylammonium bromide, add them to 1000ml of dimethylacetamide, and heat up to 150°C for reaction until theophylline basically reacts, stop the reaction, lower the temperature, filter, evaporate the filtrate to dryness under reduced pressure, and recrystallize the ethanol with a mass fraction of 95%, to obtain the intermediate 7-(2,2-dimethoxyethyl)tea alkali. The mass of the intermediate 7-(2,2-dimethoxyethyl)theophylline is 210g.
[0046] (2) Take 200g intermediate 7-(2,2-dimethoxyethyl)theophylline, 76.4g ethylene glycol, 15.8g sodium bisulfate and add it to 1200ml anisole, and the oil bath is heated to 135°C until After the raw materials have basically reacted, stop heating, lower the temperature, pour out the supernatant, and evaporate the supernatant to dryness under reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com