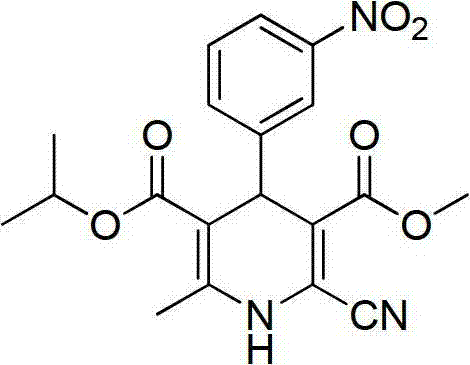
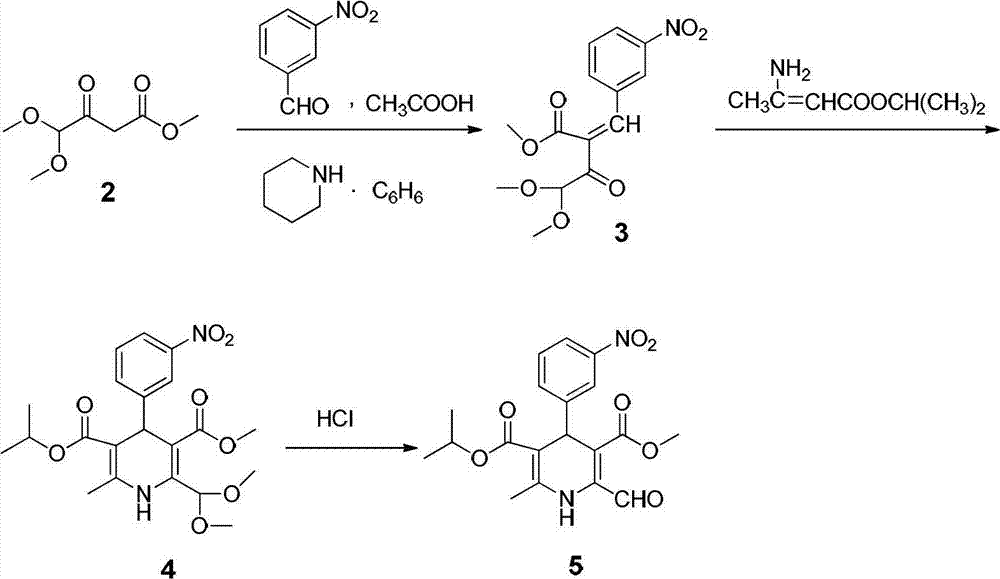
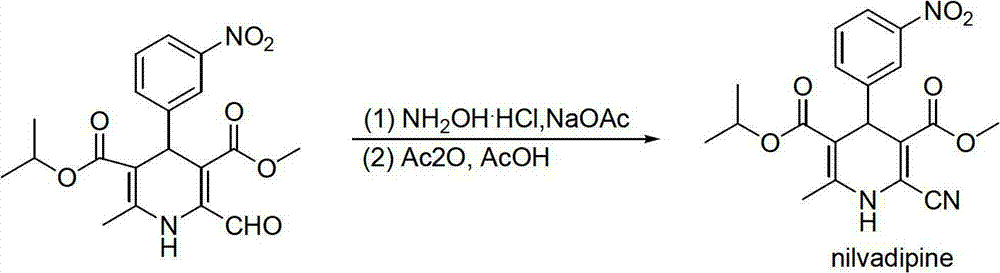Preparation method of nilvadipine intermediate
A technology of methyl and nitrophenyl, which is applied in the field of preparation of nilvadipine intermediates, can solve the problems of low purity, low yield, complicated preparation process, etc., and achieve the effect of improving purity, low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1) Put sodium hydride with a molar mass of 0.2 mol, dimethyl carbonate with a molar mass of 0.2 mol, and aceguvaldehyde dimethyl acetal with a molar mass of 0.1 mol into 50 mL of toluene to obtain a mixed solution, and reflux the mixed solution at 120°C 10h to obtain a concentrated solution. Cool the concentrated solution in an ice bath, slowly add 23mL of glacial acetic acid dropwise, then dropwise add 62mL of water, stir for 10min, stand and separate to obtain the water layer and the first organic layer, wash the water layer with 30mL of benzene and stand to separate , to obtain the second organic layer; combine the first organic layer and the second organic layer to obtain the total organic solution; wash the total organic solution with water, dry the steps, and rectify under reduced pressure to obtain 4,4-dimethoxyacetoacetate methyl ester Pure product 10.61g, yield 60.2%, bp: 69~70℃ / 3mmHg.
[0051] 2) Take 4,4-dimethoxyacetoacetate methyl ester with a molar mass o...
Embodiment 2
[0056] 1) Sodium hydride with a molar mass of 0.2 mol, dimethyl carbonate with a molar mass of 0.2 mol, and aceguvaldehyde dimethyl acetal with a molar mass of 0.1 mol were dissolved in 50 mL of benzene to obtain a mixed solution, and the mixed solution was heated at 110°C Reflux for 10h to obtain a concentrated solution. Cool the concentrated solution in an ice bath, slowly add 23mL of glacial acetic acid dropwise, then dropwise add 62mL of water, stir for 10min, stand and separate to obtain the water layer and the first organic layer, wash the water layer with 30mL of benzene and stand to separate , to obtain the second organic layer; combine the first organic layer and the second organic layer to obtain the total organic solution; the total organic solution is washed and dried, and then concentrated under reduced pressure at 3 mmHg to obtain 4,4-dimethoxy The crude methyl acetoacetate was distilled under reduced pressure to obtain 9.58g pure product, bp: 69~70℃ / 3mmHg. .
...
Embodiment 3
[0062] 1) Dissolve butyl lithium with a molar mass of 0.2 mol, dimethyl carbonate with a molar mass of 0.2 mol, and aceguvaldehyde dimethyl acetal with a molar mass of 0.1 mol in 50 ml of toluene to obtain a mixed solution, and put the mixed solution at 100°C Under reflux for 5h to obtain a concentrated solution. Cool the concentrated solution in an ice bath, slowly add 23mL of glacial acetic acid dropwise, then dropwise add 62mL of water, stir for 10min, stand and separate to obtain the water layer and the first organic layer, wash the water layer with 30mL of benzene and stand to separate , to obtain the second organic layer and combine the first organic layer and the second organic layer to obtain the total organic solution; the total organic solution was washed with water and dried, and then concentrated under reduced pressure at 3 mmHg to obtain 18.5 g of 4,4-dimethoxy The crude methyl acetoacetate was then rectified under reduced pressure to obtain 10.0 g of pure product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com