Preparation method of 1,4,7,10-tetraazadodecane
A technology of tetraazadodecane and dimethyl acetal, which is applied in the field of preparation of 1,4,7,10-tetraazadodecane, which can solve the problems of long production cycle, cumbersome operation steps and low product yield. Not very ideal and other problems, to achieve the effect of convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
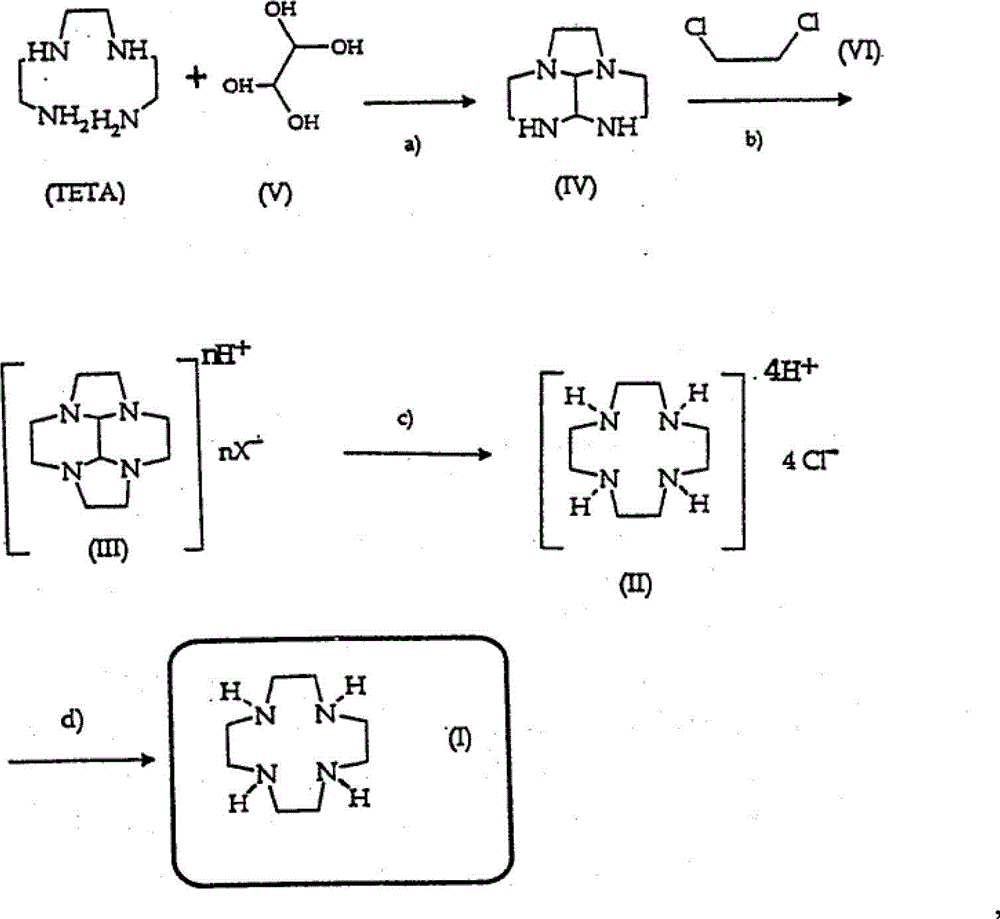
[0020] The preparation of embodiment 1 two imidazolines
[0021] In a four-necked flask equipped with a reflux condenser, a thermometer, a drying tube, and a stirrer, add TETA14.6g (0.1mol), N,N-dimethylformamide dimethyl acetal (95%) 25g (0.2mol ), 60ml of benzene, heated to 80°C, and continuously distilled off the methanol generated by the reaction, refluxed for 2 hours, steamed out an appropriate amount of benzene under reduced pressure, cooled, crystallized, and filtered to obtain 15g of white crystals, yield: 90.4%, melting point: 107 -108°C.
Embodiment 2
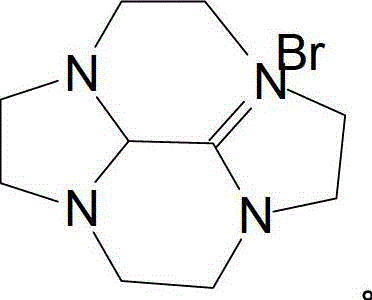
[0022] Embodiment 2 prepares bromide salt intermediate by double imidazoline ring expansion reaction
[0023] In a four-necked flask equipped with a reflux condenser, a thermometer, and a stirrer, add 11g (0.08mol) of potassium carbonate and 100ml of ethanol, heat and stir, and slowly add 6.6g (0.04mol) of bis-imidazoline dropwise at 80°C , a mixture of 7.6g (0.04mol) of 1,2-dibromoethane and 200ml of ethanol, reacted for 2 hours, cooled to 50°C, filtered off potassium carbonate, and distilled off ethanol under reduced pressure to obtain 8.4g of amber semi-solid , which is the bromide salt intermediate, add 300ml of water to dissolve in the next step, and the yield is 78.7%.
Embodiment 31
[0024] The preparation of embodiment 31,4,7,10-tetraazadodecane
[0025] In a four-neck flask equipped with a reflux condenser, a thermometer, a stirrer and a constant pressure dropping funnel, add 76g of water, stir and heat, and reflux. Add dropwise the aqueous solution 310 grams (wherein containing about 8.4g of bromine salt intermediate, 0.03mol) and 30%NaOH aqueous solution 32g (containing sodium hydroxide 2.4mol) of the bromine salt intermediate prepared according to the method of embodiment 2, dropwise, Reflux for 2 hours, filter while hot, add the filtrate to a distillation flask, distill off water under reduced pressure, cool to obtain white crystals, and filter to obtain a crude product. The crude product was dehydrated and recrystallized with toluene to obtain 4.6 g of white crystals, namely 1,4,7,10-tetraazadodecane, with a GC content of 99% and a yield of 83.9%. Melting point: 111-113°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com