Enteric capsule shell and preparation method thereof
A technology of enteric-coated capsules and solutions, which is applied in the field of enteric-coated capsule shells and its preparation, can solve the problems of capsule solubility reduction, impact on biocompatibility, impact on gelatin capsule production, etc., and achieve easy purification, low price, and easy raw materials The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
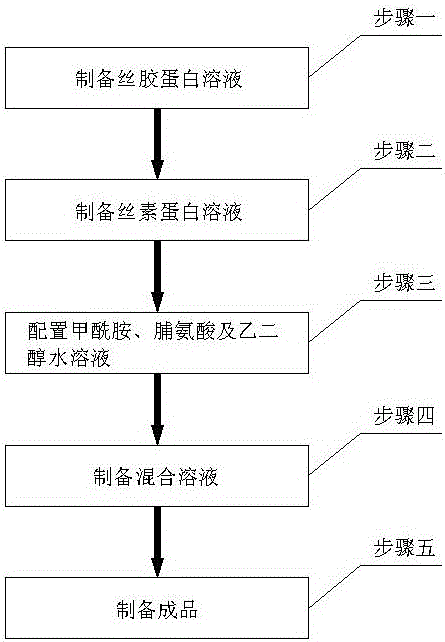
[0023] A method for preparing an enteric-coated capsule shell, comprising the following steps: Step 1: preparing a sericin solution; Step 2: preparing a silk fibroin solution; Step 3: preparing an aqueous solution of formamide, proline and ethylene glycol; Step 4 : Prepare mixed solution; Step 5: Prepare finished product;
[0024] in:
[0025] Step 1: Using silkworm cocoon shells or silkworm raw silk as raw materials, add deionized water to boil the cocoons in a boiling water bath, centrifuge and take the supernatant to obtain a sericin solution, measure its mass percentage concentration, and refrigerate in the refrigerator for later use;
[0026] Step 2: Use silkworm cocoon shells or silkworm raw silk as raw materials, degumming to remove sericin, drying, dissolving with lithium bromide, dialyzing in deionized water for 2 to 5 days at 4°C to obtain a silk fibroin solution, and measuring its mass percentage Concentration, refrigerated in the refrigerator for later use;
[00...
Embodiment 1
[0034] Step 1, boil the cleaned silkworm cocoons and deionized water in a ratio of 1:10 in a high temperature and high pressure sterilizing pot for 20 minutes, filter the cocoon shells to obtain a sericin solution;
[0035] Step 2, degumming silkworm cocoons with sodium bicarbonate-sodium carbonate buffered aqueous solution, washing and drying the silkworm cocoons to obtain degummed silk. According to the degummed silk: lithium bromide solution (9.3mol / L) = 1:20 to dissolve the degummed silk, the mixed solution is dialyzed with deionized water at 4°C for 3~4 days to obtain the silk fibroin solution;
[0036] Step 3: Take formamide, ethylene glycol and proline, configure it into an aqueous solution containing formamide, proline and ethylene glycol, and set aside;
[0037] Step 4: Mix silk fibroin, sericin, formamide, ethylene glycol, and proline into the mold at a mass ratio of 45:45:8:1:1;
[0038] Step 5: Put the mixed solution in an oven at 75°C for 1 hour, and then place i...
Embodiment 2
[0041] Step 1, cooking the cleaned silkworm cocoons and deionized water at a ratio of 1:20 in a high temperature and high pressure sterilizing pot for 1 hour, filtering the cocoon shells to obtain a sericin solution;
[0042]Step 2, degumming silkworm cocoons with sodium bicarbonate-sodium carbonate buffered aqueous solution, washing and drying the silkworm cocoons to obtain degummed silk. According to the degummed silk: lithium bromide solution (9.3mol / L) = 1:10 to dissolve the degummed silk, the mixed solution is dialyzed with deionized water at 4°C for 3~4 days to obtain the silk fibroin solution;
[0043] Step 3: Take formamide, ethylene glycol and proline, configure it into an aqueous solution containing formamide, proline and ethylene glycol, and set aside;
[0044] Step 4: Mix silk fibroin, sericin, formamide, ethylene glycol, and proline into the mold according to the mass ratio of 77:13:7:2:1;
[0045] Step 5: Put the mixed solution in an oven at 75°C for 2 hours, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com