Novel application of fusion protein TAT-DCF1
A technology of TAT-DCF1 and fusion protein, applied in peptide/protein components, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of slow development of new drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
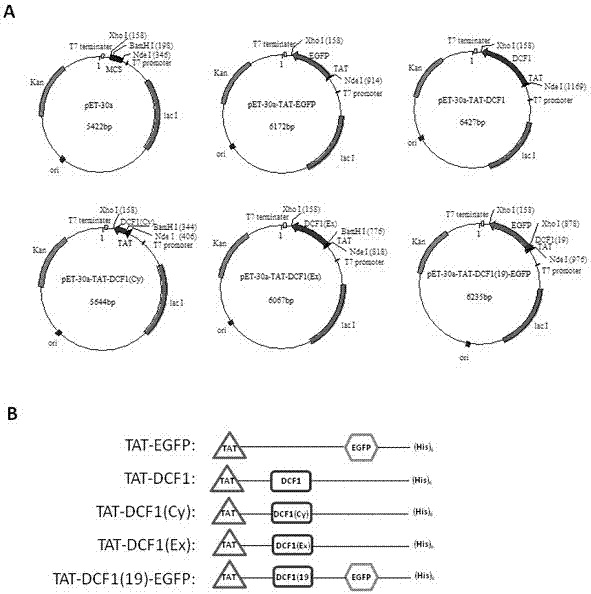
[0028] Example 1: Construction of recombinant plasmids
[0029] The experimental steps of subcloning culture and transformation of Escherichia coli refer to the standard experimental steps in the molecular cloning textbook. Using the pEGFP-N2-DCF1 plasmid as a template, DCF1 target genes of different lengths (full-length DCF1, intracellular DCF1(Cy), extracellular DCF1(Ex), autophagy fragment DCF1( 19)) and the EGFP gene, the target fragment of TAT is obtained by primer annealing. All PCR products and pET30a vector were digested with restriction enzymes Nde I and xho I performed double digestion and ligation, so that the C-terminals of the five recombinant plasmids constructed had 6xHis tags. The recombinant plasmids were named pET30a-TAT-EGFP, pET30a-TAT-DCF1, pET30a-TAT-DCF1(Cy), pET30a-TAT-DCF1(Ex), pET30a-TAT-DCF1(19)-EGFP, all plasmids were Contains (His)6 tag. The positive clones of each recombinant were selected for preliminary PCR identification and double enz...
Embodiment 2
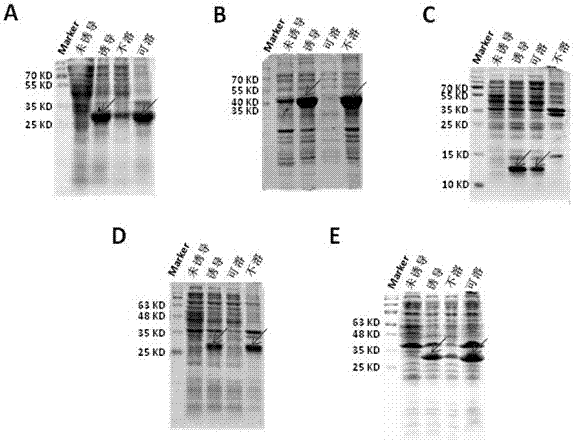
[0030] Example 2: Induced expression and soluble identification of fusion protein
[0031] 1. Transform the recombinant plasmid into RosettaTM2 (DE3) competent cells, and select transformants on LB solid culture plates containing kanamycin resistance. Pick a single colony and inoculate it in 3ml of LB liquid medium containing 50ug / ml kanamycin, and culture overnight at 37°C and 210r / min in a shaker. Draw 2.5ml of the cultured bacterial solution and inoculate it into 250ml of LB liquid medium, culture with shaking at 37°C until the OD600 in the logarithmic growth phase is about 0.6, add IPTG inducers to the solution respectively, so that the final concentrations are 0.2 and 0.4 respectively , 0.6, 0.8, 1mM, and induced for 4 hours at different temperatures (18°C, 25°C, 28°C, 37°C) to optimize the induction expression conditions. In order to analyze the induction efficiency, it is necessary to set up an uninduced control group at the same time. After the induction, centrifuge ...
Embodiment 3
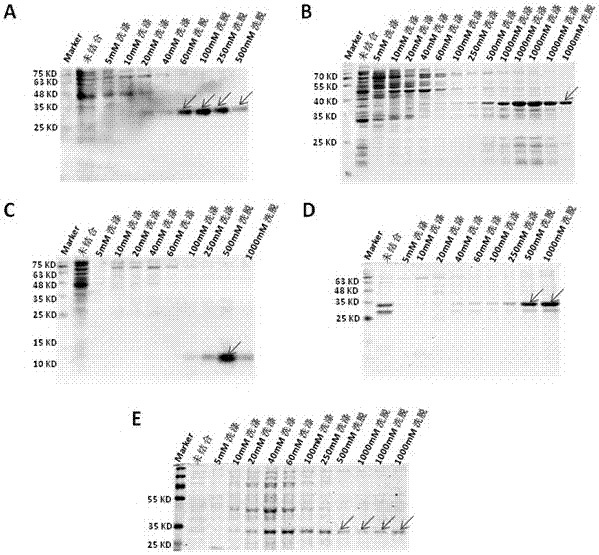
[0034] Example 3: Purification of Fusion Protein
[0035] Use Ni 2+ The fusion protein was purified by affinity chromatography. Briefly, the column was equilibrated with 5 column volumes of PBS, and the protein solution was combined with the His filler at 4°C for 2h. The column was washed sequentially with PBS washing buffer containing different concentrations of imidazole, and then the target protein was sequentially eluted with PBS elution buffer containing different concentrations of imidazole, and the purified protein was quantified with the BCA protein quantification kit. The purity of the purified protein was checked by PAGE electrophoresis stained with Coomassie blue ( image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com