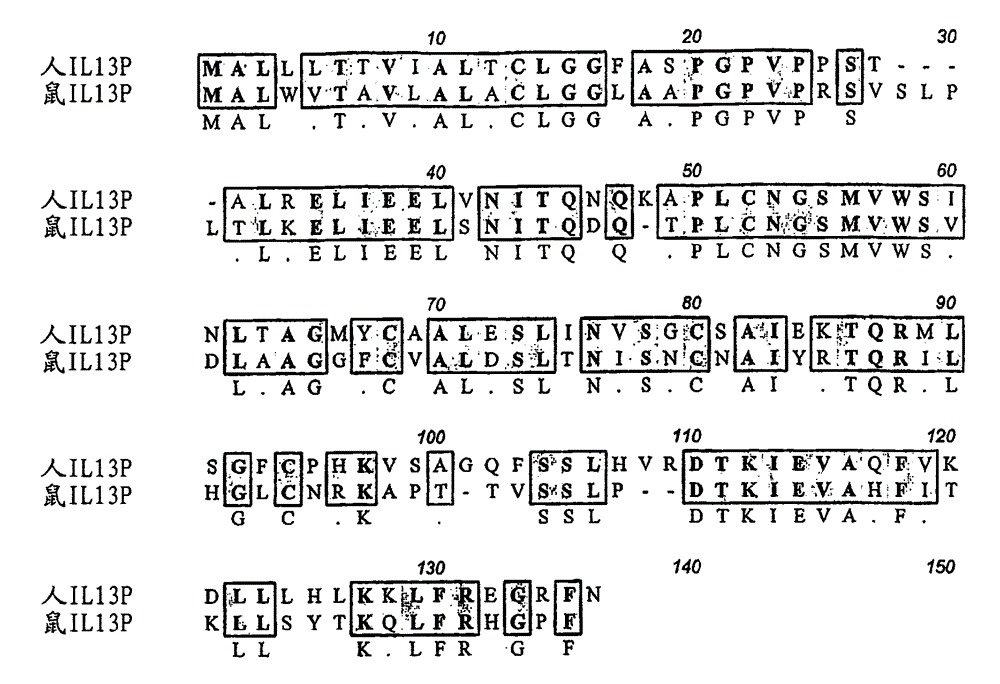
Human antibody molecules for IL-13
A human antibody, antibody technology, applied in the direction of antibodies, anti-cytokine/lymphokine/interferon immunoglobulin, anti-inflammatory agents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0270] Isolation of anti-IL-13 scFv
[0271] Repertoire of scFv antibodies
[0272] Screening was performed using a large single-chain Fv (scFv) human antibody library derived from splenic lymphocytes from 20 donors and cloned into a phagemid vector [66].
[0273] Screening of scFv
[0274] IL-13-recognizing scFvs were isolated from the phage-display library by performing a series of repeated rounds of selection on recombinant bacterial-derived human or murine IL-13 (Peprotech) essentially as described in [67]. Briefly, after incubation with the library, immobilized antigen pre-coupled to paramagnetic beads and bound phage were recovered by magnetic separation, while unbound phage were washed away. Bound phage were then rescued by the method described by Vaughan et al. [67] and by repeating the above screening process. Use different solid-phase surfaces and capture methods in different screening cycles to reduce non-specific binding. Antigens were either covalently coupled...
Embodiment 2
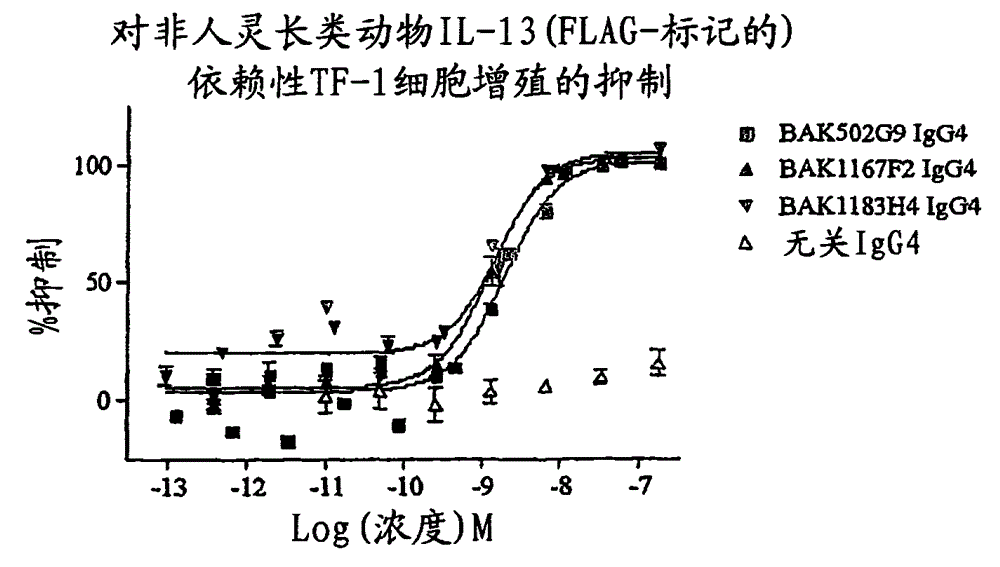
[0279] Neutralizing potency of anti-IL-13 in IL-13-dependent TF-1 cell proliferation assay
[0280] Neutralizing potency of purified scFv preparations against human and murine IL-13 bioactivity was assessed by TF-1 cell proliferation assay. Purified scFv preparations were prepared as described in Example 3 of WO01 / 66754. Protein concentrations of purified scFv preparations were determined using the BCA method (Pierce). TF-1 is a human myeloblastic cell line established from patients with erythroleukemia [68]. The survival and proliferation of the TF-1 cell line is factor-dependent. In this regard, TF-1 cells are responsive to human or murine IL-13 [69] and can be maintained in media containing human GM-CSF (4 ng / ml, R&D Systems). Inhibition of IL-13-dependent proliferation was determined by measuring the reduction of deuterium-labeled thymidine incorporated into newly synthesized DNA of dividing cells.
[0281] TF-1 Cell Assay Protocol
[0282] TF-1 cells were obtained fr...
Embodiment 3
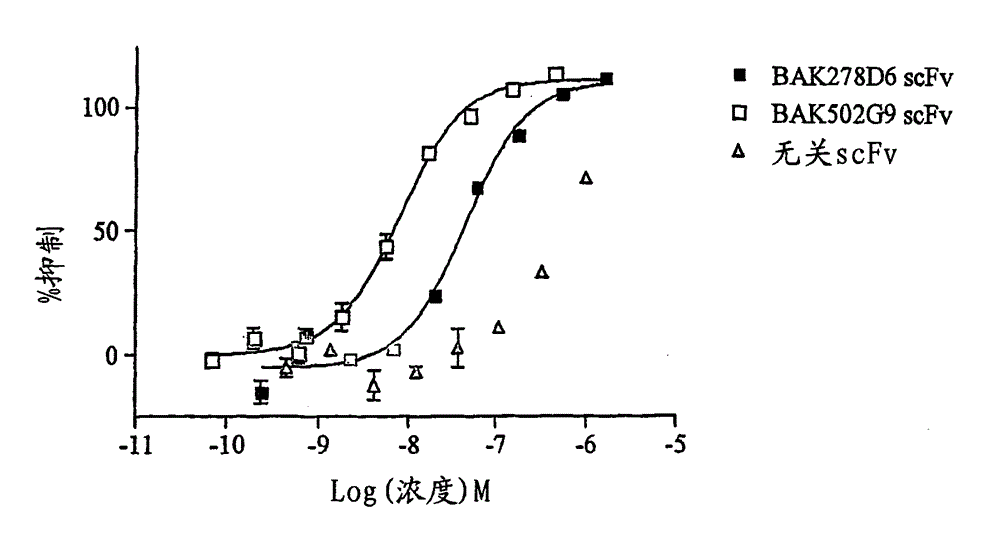
[0286] Neutralizing potency of the lead clone obtained by optimizing parental clone heavy chain CDR3 in IL-13-dependent TF-1 cell proliferation assay
[0287] Osbourn et al. [70] demonstrated that directed mutagenesis of residues within the CDR3 of the heavy chain significantly increased antibody affinity. The scFv repertoire was screened as described in Example 1, wherein residues within the heavy chain CDR3 of BAK278D6 (SEQ ID NO: 6), BAK167A11 (SEQ ID NO: 57) had been randomized by mutagenesis. Unique clones among the products obtained from this screen were identified by DNA sequencing, and their neutralizing potency as scFv in the TF-1 cell proliferation assay was evaluated as described in Example 2.
[0288] result
[0289] Both pedigrees showed significant increases in potency. The most effective clones in the BAK167A11 lineage are BAK615E3, BAK612B5 and BAK582F7, and the ICs of the three as scFv against 25ng / ml human IL-13 in the TF-1 cell proliferation assay 50 3nM ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com