A kind of anti-tumor protein and its application
A protein, anti-cancer technology, applied in anti-tumor protein and its application field, can solve the problems of difficult protein, huge difficulty in extracting protein, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Construction of embodiment 1PvTrag17 recombinant plasmid
[0022] Prokaryotic expression plasmid pET28a(+), host strain BL21(DE3) and IPTG for induction were purchased from Beijing Quanshijin Biotechnology Co., Ltd.; restriction enzymes, T4 DNA ligase, pfu DNA polymerase and dNTP were purchased from TakaRa Corporation. The synthesis of primers and nucleotide sequence sequencing were completed by Suzhou Jinweizhi Biotechnology Co., Ltd. Agarose affinity medium nickel column (Ni) was purchased from QIAGEN. His-Taq tag antibody was purchased from Cell Signaling Technology Company.
[0023] Design primers to obtain the gene sequence of P. vivax PvTrag17 protein by PCR. The primers are as follows: SEQ ID NO.3: GGATCCATGGAACTAAAAAGCCAATATG; SEQ ID NO.4: CTCGAGTGAGTCATTATCTGTGCTCAC, where GGATCC is the restriction site BamHI of SEQ ID NO.3; CTCGAG is Restriction site XhoI of SEQ ID NO.4.
[0024] Using the Plasmodium ovale genome as a template, the PvTrag17 gene sequence w...
Embodiment 2
[0027] Embodiment 2 Expression of recombinant protein PvTrag17
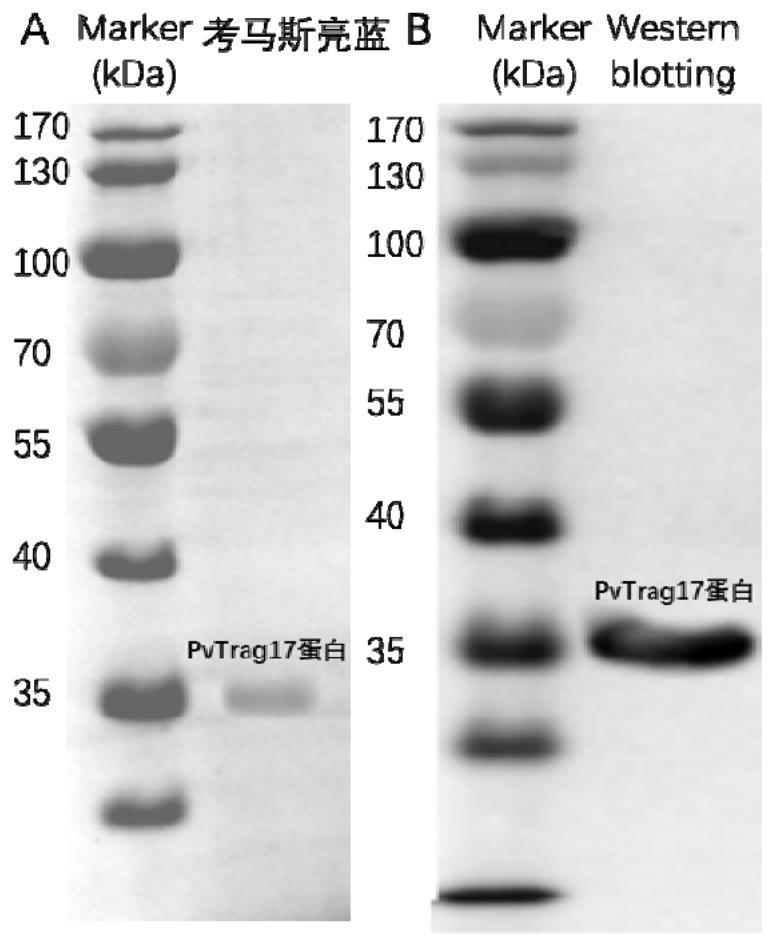
[0028] Inoculate 5ml of LB medium containing kanamycin with the positive single clone with correct sequencing, culture overnight at 37°C, inoculate the bacterial liquid into 500ml fresh LB medium containing kanamycin, when OD 600 When it is 0.6-0.8, add 1mmol / L IPTG, induce 8h. The induced PvTrag17 was ultrasonically disrupted and lysed, and the 10% SDS-PAGE electrophoresis analysis showed that the PvTrag17 protein was mainly located in the inclusion body, and the molecular weight was consistent with the expectation.
[0029] Dissolve inclusion bodies with 8M urea to release PvTrag17 protein, which has a His-tag tag at the carbon terminus. Therefore, use GE’s His-tag nickel column and carry out Ni 2+ Affinity chromatography purification. The protein was purified with different concentrations of imidazole. The concentrations of imidazole were 20mM, 50mM, 100mM, 150mM and 250mM. The protein washed with 150mM imid...
Embodiment 3
[0031] Example 3 Identification of anti-tumor activity of PvTrag17 protein
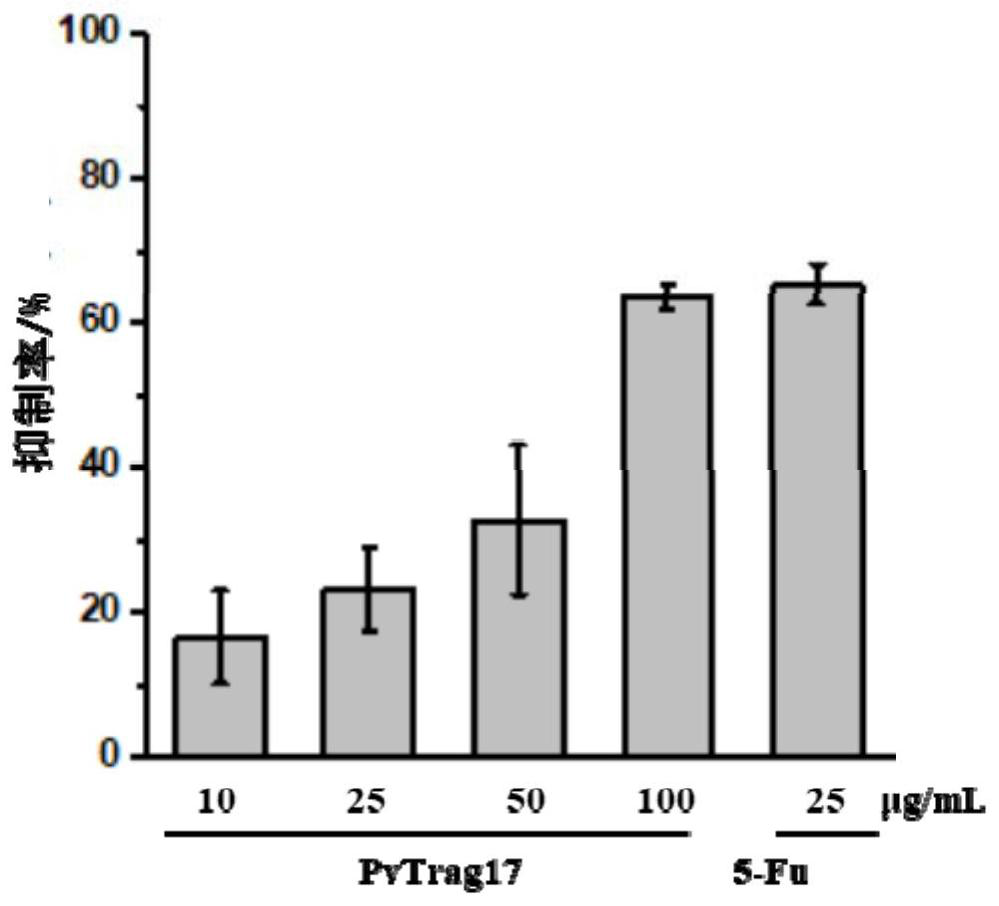
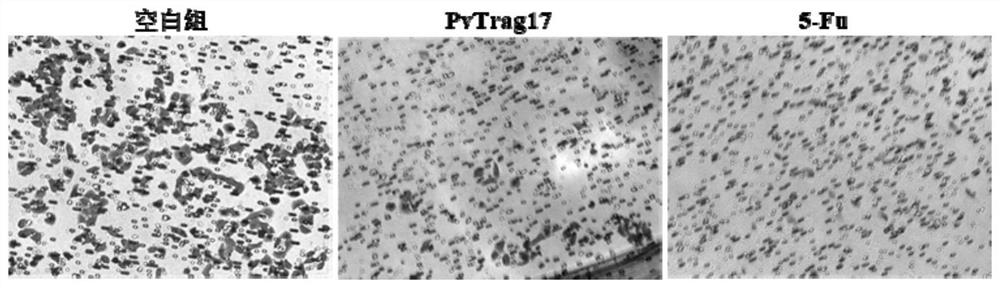
[0032] (1) CCK8 method to detect the inhibitory effect of PvTrag17 on the proliferation of tumor cells
[0033] Take HepG2 in the logarithmic phase and use DMEM medium containing 10% FBS to adjust the cell concentration to 5×10 4 / mL, inoculated in 96-well plate with 100 μL per well, placed in 5% CO 2 , cultured in a 37°C incubator for 6 hours, discarded the supernatant after the cells adhered to the wall, washed with PBS buffer to remove non-adhered cells. 100 μL of drugs with different concentrations (10, 50, 100 μg / mL) were added to the sample group, and an equal volume of medium was added to the blank group. After continuing to culture for 24 hours, add 10 μL CCK8 solution to each well, continue to culture in the incubator for 2 hours, and measure the absorbance at 450 nm. A dose of 25 μg / mL antineoplastic drug 5-Fu was used as the control. Cell proliferation rate (%)=experimental group A450 / c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com