Octreotide acetate preparation and preparation method thereof
A technology for octreotide acetate and preparation, which is applied in the field of octreotide acetate proliposome preparation and preparation, and can solve the problems of difficulty in achieving high encapsulation efficiency and lack of visibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
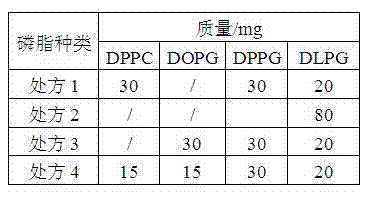
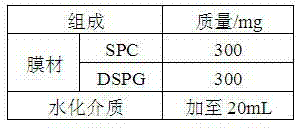
[0040] Example 1 Preparation of octreotide acetate liposomes by tert-butanol-water co-solvent freeze-drying method (1:60)
[0041] prescription
[0042] SPC 300mg
[0043] DSPG 300mg
[0044] Preparation:
[0045] 1) Dissolution: Weigh the prescribed amount of SPC and DSPG into a container, add 8 mL of tert-butanol, dissolve in a water bath at 65°C, add 18 mL of octreotide acetate solution containing 10% sucrose (concentration: 0.33 mg / mL), and continue adding in a water bath Until it is completely dissolved, until the solution is clear and transparent, dispense it.
[0046] 2) Freeze-drying: The freeze-drying curve is as follows: Pre-freeze at -74°C for 8 hours → vacuumize at -35°C for 1 hour, and finally reach a vacuum of about 5 Pa → increase the temperature to -25°C and keep for 12 hours (sublimation drying) → The temperature was raised to 20°C and kept for 3 hours (analysis and drying) to obtain proliposomes.
[0047] 3) Reconstitution: Add 2.5 mL of 5% glucose solu...
Embodiment 2
[0049] Example 2 (1:15)
[0050] EPC 300mg
[0051] DPPG 66.7mg
[0052] Preparation:
[0053] 1) Dissolution: Weigh the prescribed amount of EPC and DPPG into a container, add 4 mL of tert-butanol, dissolve in a 65°C water bath, add 18 mL of acetic acid containing 5% sucrose (w / v) and 5% lactose (w / v) Octreotide solution (concentration: 0.33 mg / mL), continue to add in water bath until it is completely dissolved, until the solution is clear and transparent, then dispense it.
[0054] 2) Freeze-drying: The freeze-drying curve is as follows: Pre-freeze at -74°C for 8 hours → vacuumize at -35°C for 1 hour, and finally reach a vacuum of about 10 Pa → increase the temperature to -25°C and keep for 12 hours (sublimation drying) → The temperature was raised to 20°C and kept for 4 hours (analysis and drying) to obtain proliposomes.
[0055] 3) Reconstitution: Add 2.5 mL of 5% glucose solution to the precursor liposome, disperse and redissolve, and obtain the octreotide acetate l...
Embodiment 3
[0058] prescription
[0059] EPC 260mg
[0060] DPPG 30mg
[0061] DOPG 30mg
[0062] Preparation:
[0063] 1) Dissolution: Weigh the prescribed amount of EPC, DPPG and DOPG into a container, add 4 mL of tert-butanol, dissolve in a water bath at 65°C, add 18 mL of octreotide acetate solution containing 5% sucrose (w / v) (concentration: 0.33 mg / mL), continue to add in a water bath until completely dissolved, until the solution is clear and transparent, and dispense.
[0064] 2) Freeze-drying: The freeze-drying curve is as follows: Pre-freeze at -74°C for 8 hours → vacuumize at -35°C for 1 hour, and finally reach a vacuum of about 5 Pa → increase the temperature to -25°C and keep for 12 hours (sublimation drying) → The temperature was raised to 20°C and kept for 5 hours (analysis and drying) to obtain proliposomes.
[0065] 3) Reconstitution: Add 2.5 mL of 5% glucose solution to the precursor liposome, disperse and redissolve, and obtain the octreotide acetate liposome prep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com